Caymen finally got deschloroketamine available as a standard recently, so we got some and were able to confirm that two previously unidentified samples were deschloroketamine.
See EcstasyData 4350 and EcstasyData 4070.
Thanks Cayman and DDL!
Caymen finally got deschloroketamine available as a standard recently, so we got some and were able to confirm that two previously unidentified samples were deschloroketamine.
See EcstasyData 4350 and EcstasyData 4070.
Thanks Cayman and DDL!
In April 2015, we received a sample that was sold as 6-MAPB, but the lab tentatively identified as most likely to be 6-APDB. This week, the lab took another look at the sample with some new standards and using more updated Mass Spectrum databases.
After spending quite a bit of time on it, their new identification (not 100% match to a standard DDL has, but a close library match) has changed to 5-APDB, rather than the previous 6-APDB. Here’s what the lab wrote describing that process:
I reanalyzed 40900034. The best match is now to 5-APDB. It’s complicated, so here’s a little background.
There are four APDB compounds, the 4-APDB, 5-APDB, 6-APDB, and 7-APDB. They all elute very early according to Cayman spectras, but in this order, 5, 6, 7, and then 4 at a later time. So, it was determined that based on the ion ratios, 40900034 was CLOSEST and now BEST match to 5-APDB. The GCMS output spectras of 5,6,7 in comparison to the sample have been attached, just for curiosity.
[ Erowid: these are now attached to the ecstasydata entry: ID : 3276. ]
Also, it was interesting that the 6-APDB standard received from Cayman produced an extra minor peak, whereas only one peak is shown in Cayman’s database. Not sure there. Possible contamination of the standard we were sent? Or 6-APDB produces two peaks. ( I ran the standard three different times to confirm). GCMS output have also been attached.
I will be developing a new method to help separate and better identified compounds with functional groups at a different carbons for APDB and the many other new drugs.
We received a sample this month that was sold as 6-APB but the lab identified by library match to be 5-MAPDB. A “library match” is not 100% guaranteed to be a positive identification because it means that the mass spectrum was consistent with a published mass spectrum from an established library. What library matches are missing is exactly the gas chromotagraphic timing and the exact minor details around the edges of the spectrum that are unique to each type of analytic equipment.
To confirm the identification, we ordered analytical standards from Cayman for 5-MAPDB. The lab ran the standard and then compared them against the submitted sample (ID:3688) and found they matched exactly, confirming their library match that the sample is 5-MAPDB.
Short Summary: Marquis, Mecke, Mandelin, and Simons are not reliable tools for confirming or disproving the presence of LSD in blotter, liquid, gel, or tablet forms.
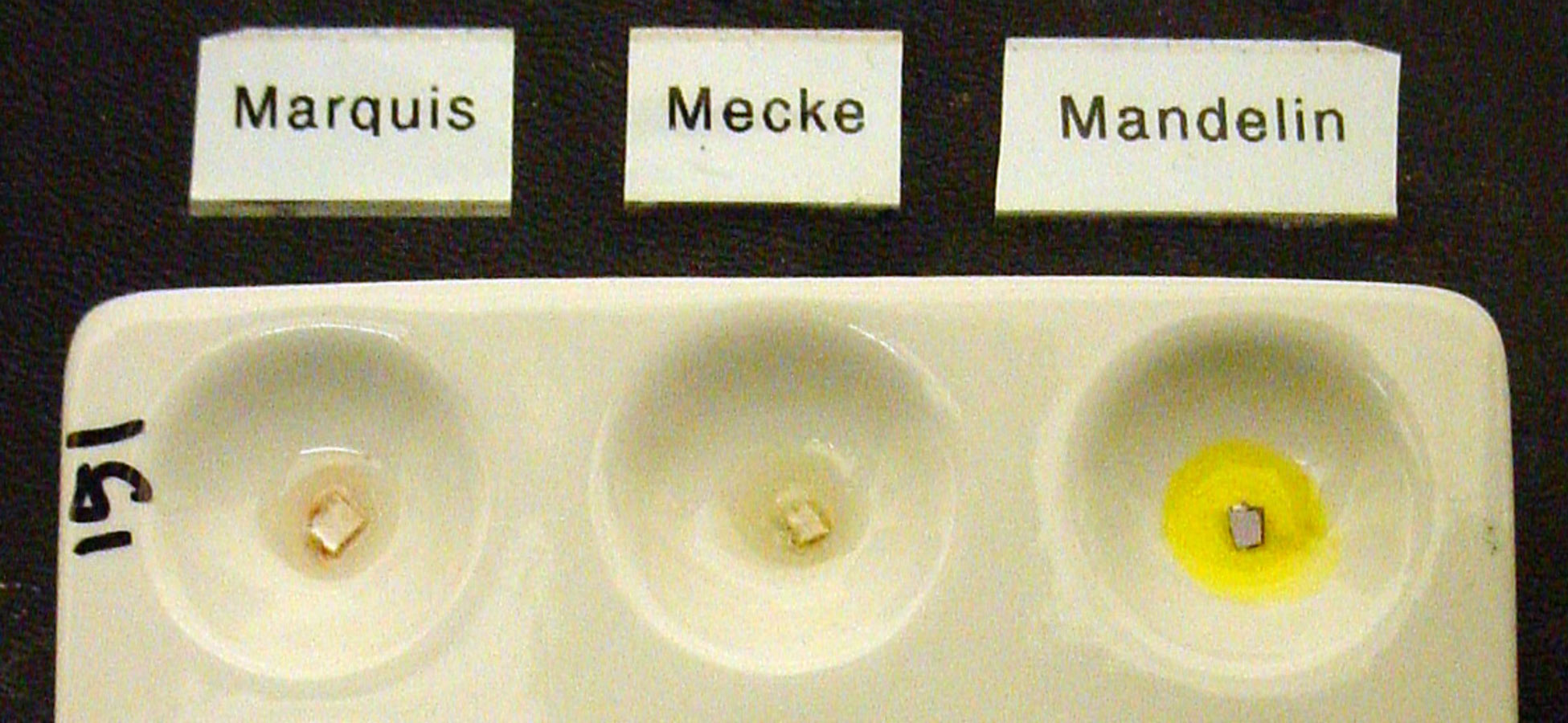
Long Version: We get asked a lot about how specific drugs react to the various drug-detection field tests like Marquis, Mecke, Mandelin, Simons, Robadope, Ehrlich’s, etc. In most cases, it’s a matter of getting some of the pure target compound, a fresh set of reagents, and doing a little testing, photographing, and documentation..
However, with drugs active below 1mg, such as LSD, this may not be so simple. Because the amount of target compound is often very small, the reactions can be altered, slowed, or blocked by tiny amounts of other substances present. In the case of LSD blotter paper, where the amount of LSD on a 1/4″ square (6mmx6mm) is usually at or below 100 micrograms, the paper and the ink on the paper are far more likely to be the cause of a color change than the LSD itself. With liquid LSD, the alcohol carrier can dilute the response enough that no color change is visible. Thus, a color change or the lack of color change can be due to the form in which the substance is being tested.
LSD is said to create an olive green or black reaction with a Marquis reagent test. Organizations that sell reagent tests such as Dancesafe and Bunk Police report that LSD has an olive-black reaction with a Marquis test. This may be based upon sources such as this Department of Justice article stating that LSD causes an “Olive black” result. [ Fatah A. “Color Test Reagents/Kits for Preliminary Identification of Drugs of Abuse” (2000) ].
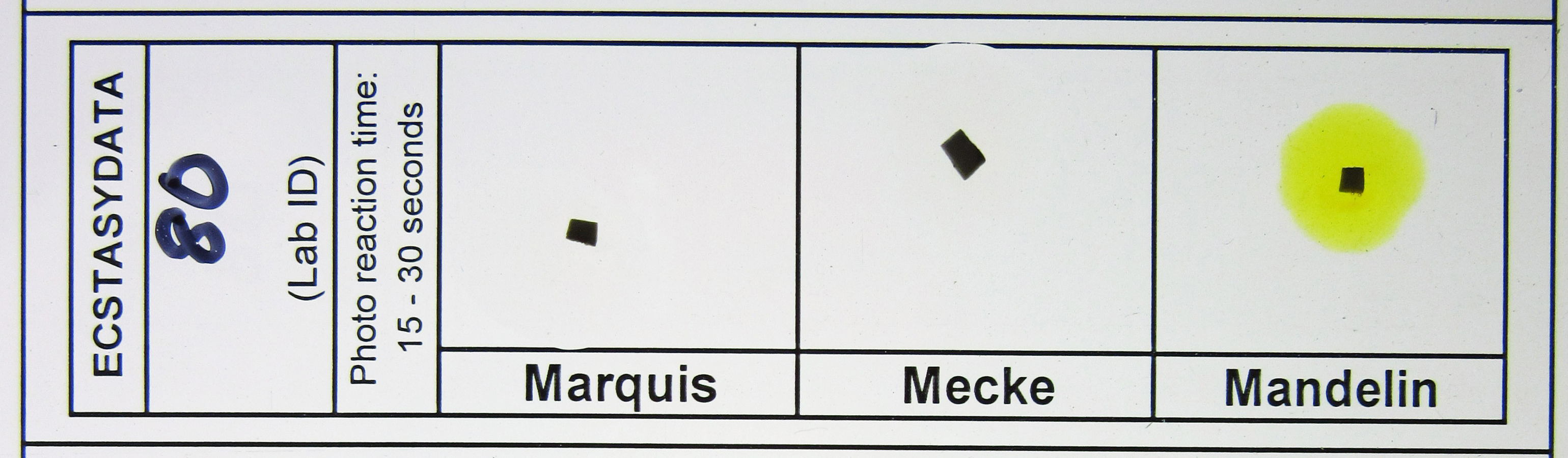
However, the results of our own lab’s field tests on samples confirmed to contain LSD using GC/MS show varying responses to Marquis and other field tests. When tested with a Marquis reagent, most of these samples showed no reaction or only a very slight reaction and none produced an olive green or black reaction. For examples of these test results see Figure 1 and Figure 2. Furthermore, the blotter paper itself—not the LSD—may be causing these slight reactions.
You can see a list of numerous LSD samples on EcstasyData along with their field test color changes here:
http://www.ecstasydata.org/results.php?start=0&search_field=substance&s=lsd&detail=true
Despite claims that LSD produces an olive green or black reaction to Marquis, evidence collected and published through EcstasyData does not support this. Furthermore, a 1979 paper by by Johns et al is consistent with these EcstasyData results. The researchers tested a wide variety of drugs from that era with nine different reagent tests, including Marquis and Ehrlich (Table 1). This paper reports that LSD only reacts with Ehrlich and not with any of the other reagents. Interestingly, the researchers used dry LSD from Sandoz Laboratories. Other publications claiming that LSD produces olive green or black reactions to Marquis do not report the source or form (i.e. liquid, crystal, or blotter) of the LSD tested. The results of the Johns et al., study suggests that confirmed pure crystal LSD has no reaction with a Marquis test. Below is a summary of the results:
| Compound | Reagent test | Color reaction | Testing surface |
|---|---|---|---|
| LSD | Marquis | no reaction | Porcelain |
| LSD | Mecke | no reaction | Porcelain |
| LSD | Madelin | no reaction | Porcelain |
| LSD | Cobalt thiocyanate | no reaction | Porcelain |
| LSD | Dille-Koppanyi | no reaction | Porcelain |
| LSD | Ehrlich | purple | Porcelain |
| LSD | Froehde | no reaction | Porcelain |
| LSD | Sulfuric acid | no reaction | Porcelain |
| LSD | Nitric acid | no reaction | Porcelain |
Although other sources say that LSD causes an olive-black reaction with a Marquis reagent test, this study, along with the EcstasyData results, suggests that LSD may not cause an olive-black reaction with a Marquis test—at least not on blotter paper in the amounts it is commonly sold. Based on this evidence, the Marquis is not a reliable for test for LSD. On the other hand, the Ehrlich may be an alternative test for this purpose based on the findings published by Johns et al. The Ehrlich reagent produces a color change when LSD or another indole is present. However, like all reagent tests, an Ehrlich is not a confirming test, it is just another in a wide array of rule in / rule out tests that can help confirm or deny the presence of LSD in a given sample.
Thanks to W and Shaolin at the DMT Nexus for their work on this issue.
A visitor asked about result 3669. This result was determined by DDL via GC/MS to contain primarily MDMA but also a trace amount of Methylsulfonylmethane (MSM). MSM has some practical applications in the final crystallization of some recreational stimulants (notably methamphetamine) but could also be used as a bulking agent to boost retail profits.
The visitor wanted to know how sure we are that the MSM was only a trace and not a more major component of the sample. Here’s an answer from our lab trying to address the issue:
There are numerous complications when asking how certain the ‘trace’ finding is for MSM in the testing process.
First, DDL has never done a quantitative analysis of MSM using our testing process. So our methods for analyzing MSM have not been validated by our team.
It could be that it might take a little more MSM to show up compare to say meth or MDMA, since MSM is a much smaller compound. We don’t know this for certain and would need to spend time verifying it if this is important to the project.
Second, MSM is easily detected and seen. We know it shows up we have detected it in many samples over the years.
Third, although we do not know for certain how a known quantity of MSM mixed uniformly with a known quantity of MDMA would show up in our GC/MS, it is hard to imagine the trace we saw with this sample representing more than a tiny amount in the actual sample analyzed.
Fourth, process issues it is possible that the sample submitted contained more MSM proportionally than we reported due to process issues. It could be that the sample might not be homogenized. If a baggie or capsule is filled with MSM and Meth, but wasn’t thoroughly mixed when filled, and when we open the capsule a pour a good representative amount to use, maybe there’s more MSM powder at the end of the capsule. Or perhaps MSM is not homogenized on a sample tablet when a good portion is scraped off or a portion broken off and crushed for analysis.
If we were primarily concerned about homogenization, we would dissolve the entire sample in a solvent, but we don’t do this for several reasons. We don’t want to use up the whole sample on the first attempt because a sample may not dissolve perfectly in the first solvent we choose or we may need to re-evaluate the analytical process from scratch and we would not want to add a solvent to all of the powder if we can avoid it. There are more reasons to keep the original solid sample than there are to homogenize the whole thing.
Fifth, one thing is certain, we can’t be certain. The way each drug appears using each analytical technique different. Because of this, quantitative analysis of drugs in forensic toxicology typically involves very technical development of validated processes using the drugs in their deuterated forms as internal standards.
So, there are numerous variables that may explain it only being trace relative to other drugs. Most likely, the sample only contained a tiny amount of MSM, but without further analysis this point, there’s no way to be certain.
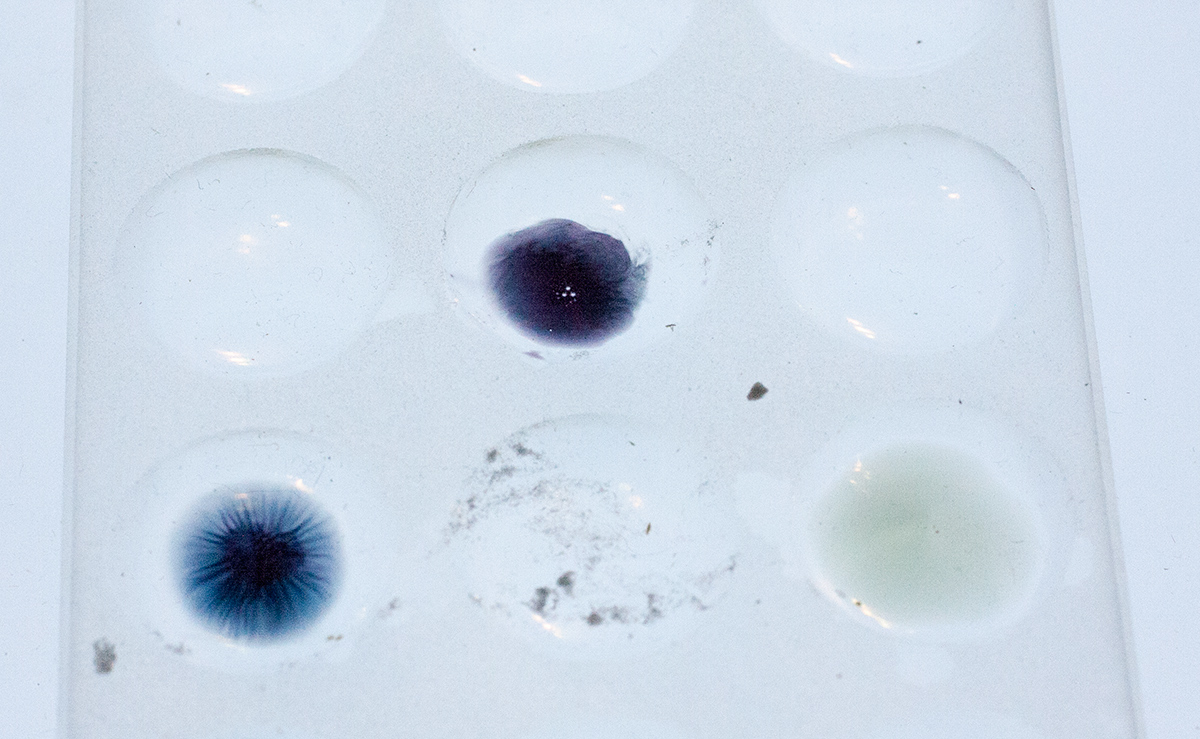
We’ve been trying to come up with better ways to do and photograph the spot plate reagent field tests for the variety of chemicals. Both the ones sold as ecstasy/molly and the wide variety of substances and pharmaceuticals that people send in for analysis.
The solid ceramic spot plate we’ve been using for the last 15 years makes it so the colors are hard to see in darker reactions.
So we’ve been hunting down translucent glass plates but haven’t found many. We would like one that we can use AND recommend to Erowid visitors around the world they can buy for a reasonable price.
Here are some photos comparing the translucent spot plate with the opaque one.
Old spot plate:

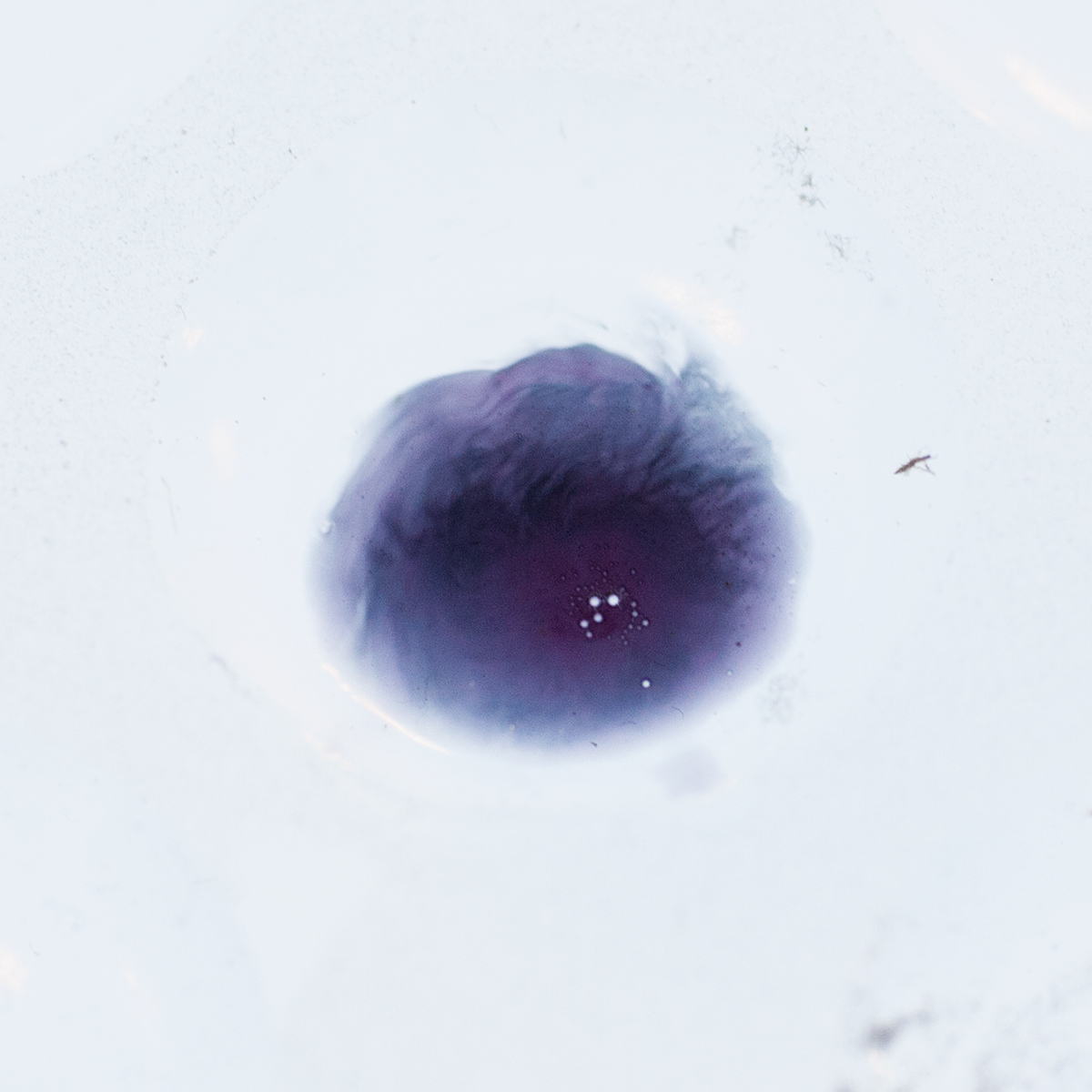
Perhaps the most interesting is that all three of these color spots are Marquis + the same chemical. The right hand spot is the oldest (over an hour since drop), the left one is maybe 30+ minutes, and the top middle one was dropped within a minute. This chemical starts off as a grey purple, to a very dark nice blue purple, then goes to a dark blue, then winds up in this greenish gray color.