— by: earth, Sylvia, Fire, Jurek, and anonymous experts
Here’s a peek into how Erowid works with a network of drug-checking experts around the world work. Just another day at DrugsData. :]
On June 30, we published the test results for a sample of 1P-LSD blotter (dd10683), confirming the presence of 1P-LSD.
On July 12, Jurek from protestkit.eu, a Polish harm reduction and field reagent specialist, inquired about this sample, noting that the Ehrlich reagent photo showed an unexpected purple reaction. Jurek pointed out that 1P-LSD isn’t known to result in a purple color in the presence of Ehrlich reagent, helping to differentiate it from LSD-25, which does cause a purple color change with Ehrlich reagent.
We discussed this with our lab and learned that there was a small GC peak they had not initially reported in the results: inactive salts and inks on blotter do not always get reported due to DEA-imposed limitations.
Given the unexpected Ehrlich reaction, we published the spectrum for the unidentified chemical and added it to the results as a second chemical present in the sample.
A chemist in the Erowid Expert Network identified the unknown chemical as tryptamine, so we ordered a lab standard for tryptamine and found that it was a perfect match via GC/MS.
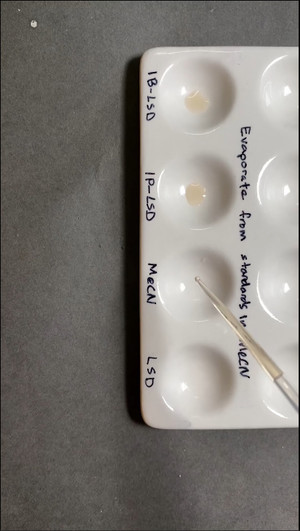
Further, DrugsData’s lab did side-by-side comparison in a ceramic well plate of lab standards for 1B-LSD, 1P-LSD, and LSD-25. The third of four wells is the ‘blank’ labeled MeCN (acetonitrile) which was the solvent used to dissolve each of the ergoloid standards (1B-LSD, 1P-LSD, LSD-25). Ehrlich reagent was applied to each, demonstrating that neither 1B-LSD nor 1P-LSD turn purple with Ehrlich, where LSD-25 does.
So the mystery of the the unexpected Ehlrich reaction for this 1P-LSD blotter is resolved, but the reason why someone added tryptamine to 1P-LSD blotter is still open. We all guess the goal was to be able to sell the 1P-LSD blotter as LSD-25, and that adding tryptamine to the 1P-LSD will result in reagent reactions consistent with LSD-25.
This is the first time Erowid has seen this type of adulteration of non-LSD ergoloids with the chemical tryptamine.
The image below is a link to a video of the reagent test:
Then, a photo of lab-grade tryptamine reacted with Ehrlich. A strong purple color: