For over forty years, methamphetamine-using communities have been speculating why some batches of meth seem qualitatively different than others. When we started working in this field in the 1990s, the claim was that l-meth, solvents, and synthesis impurities were the culprits.
Since around 2010, the DEA’s policies have resulted in most methamphetamine inside the United States coming from large manufacturers outside the US. In the ensuing years, some have claimed that the unpleasant effects of street meth in the US are a result of meth containing N-Isopropylbenzylamine (isopropylbenzylamine; N-IPBA; “N-iso”) [PubChem]. It is a common enough claim that many skeptics have called N-iso the new meth boogeyman.

The most common claims about isopropylbenzylamine are that it is present in combination with meth, and causes more paranoia, psychotic ideation, and worse hangovers. It’s also blamed for effects atyptical for stimulants, such as lethargy and “brain fog”, 1-3 days into a meth-using session.
We’ve been asked about N-iso many times between 2019 and 2022. We purchased the certified reference lab standard for isopropylbenzylamine twice in the last two years in order to run experiments to check our methods. We have repeatedly confirmed that zero (0) meth samples analyzed by DrugsData to date have contained it:
https://drugsdata.org/results.php?substance1=94&substance2=2036
Erowid’s DrugsData reported those findings to the people submitting the samples in question, and on the corresponding entries on DrugsData.org. Up until now, we haven’t specifically pointed out publicly that multiple submitters have claimed that isopropylbenzylamine could be present in their methamphetamine and that our findings have not substantiated their claims.
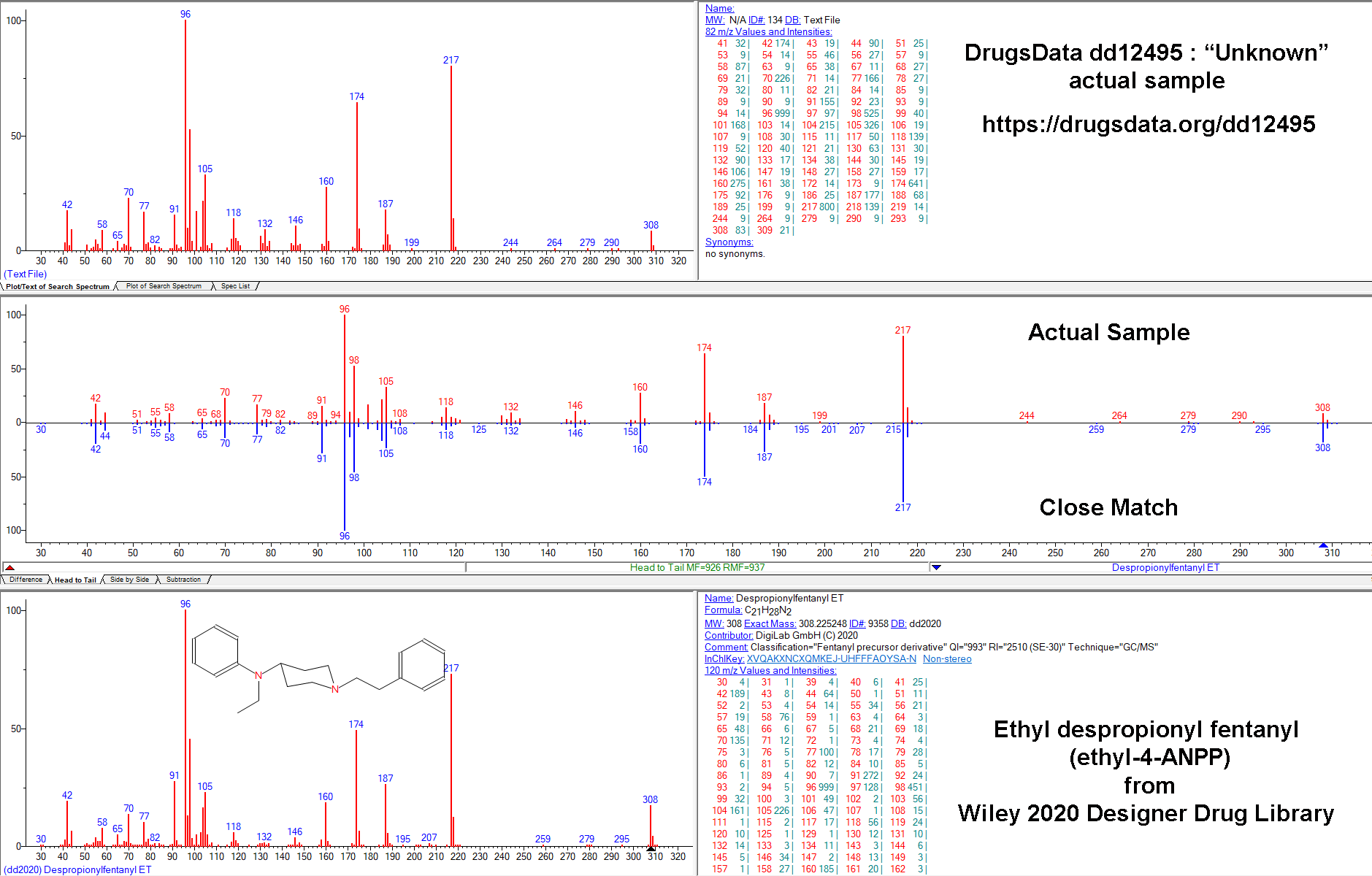
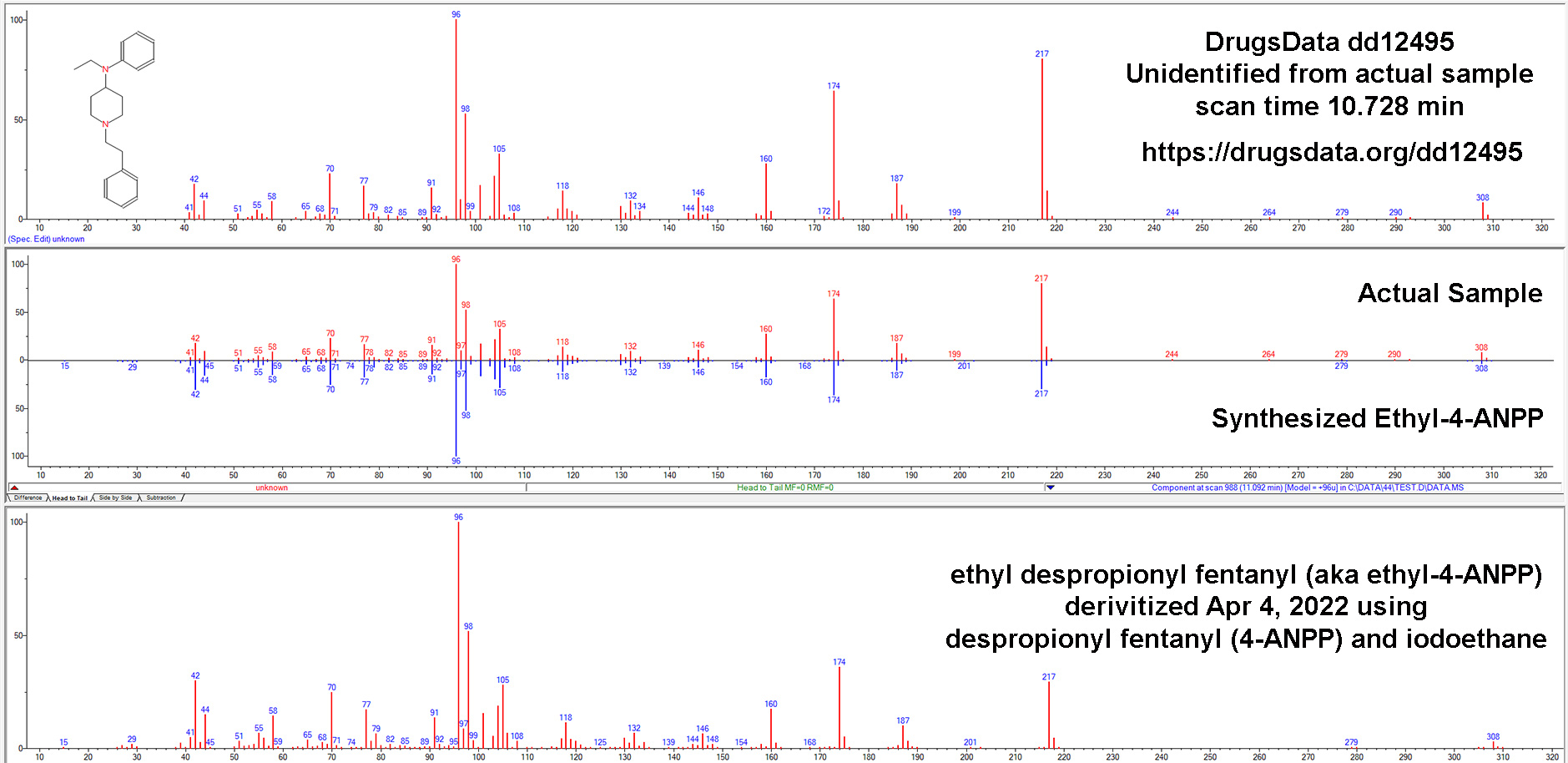
Our results caused others to ask us more and more pointed questions about how sure we were that N-iso wasn’t in the meth we tested. So we dug deeper. After re-analyzing and examining the GC/MS data for more than a dozen samples submitted to DrugsData for which the submitter was certain their meth sample contained isopropylbenzylamine, we’ve still seen no results where this is the case.
Out of 271 samples containing methamphetamine analyzed between January 2019 and July 2022, none contained isopropylbenzylamine:
https://drugsdata.org/results.php?s=methamphetamine&search_field=substance&y1=2019&y2=2022
Re-examining Our Findings
In late 2020, we ran several samples of alleged meth + N-iso and they came back meth only. We bought the reference standard and tried slightly-modified procedures using this standard. It seemed to us that meth + isopropylbenzylamine resolved easily via GC/MS. That is to say, meth and N-iso were easily differentiated in our lab. They are close, but we deal with much harder problems all the time.
In May 2022, D.M. contacted us to point out a paper that said it is possible that mixed samples containing both meth and isopropylbenzylamine could cause analysis to fail to see one or the other. Essentially, they claimed our previous findings might be wrong because one of the two could hide inside the GC curve of the other and then elute (come out) at such a similar time into the Mass Spectrometer that our software would report only one of the two chemicals.
That paper is Luo Y, et al. (2021) “Simultaneous Determination of Methamphetamine and Its Isomer N-Isopropylbenzylamine in Forensic Samples by Using a Modified LC-ESI-MS/MS Method”. (ResearchGate Link) The authors write:
“However, the two compounds [methamphetamine (MA) N-isopropylbenzylamine ([N-IPBA]) ] were hard to be effectively discriminated by GC/MS when there was a large concentration difference between them. Because the retention times for MA and [N-IPBA] chromatographic separation were very close due to their high similar chemical structure, the compound with high concentration would interfere with another one with low concentration as the two compounds yield similar ion fragments for detection [25].”
Note the relevant claim in their paper is actually cited to someone else and is not something these authors themselves demonstrate in their article. The original citation (Xuan J et al 2015) is a paper in a Chinese journal that we’ve been unable to locate.
Given this new, reasonably specific claim from a 2021 paper, despite having done it before, we purchased a new isopropylbenzylamine reference standard, this time from a different chemical supplier. Unsurprisingly, it matched exactly the previous standard and also matched the GC/MS data in the main public/research/commercial/forensic libraries.
A Series of Experiments
We mixed pure d-meth with N-IPBA at 1:1, 1:10, 1:100, 10:1, and 100:1 ratios. In all of the conditions, our setup showed isopropylbenzylamine as clearly distinct from methamphetamine. They would not be mistaken for one another or lost, even way below 1:100. Our standard procedure involves methanol run through an Agilent GC/MS 5973 MSD with the GC column being an HP-5ms Ultra inert (5%-phenyl)-methylpolysiloxane. Unless you’re a lab tech, that won’t mean anything to you, but it’s a fairly normal setup for doing drug work like this and it’s well suited to analyzing chemicals of this type.
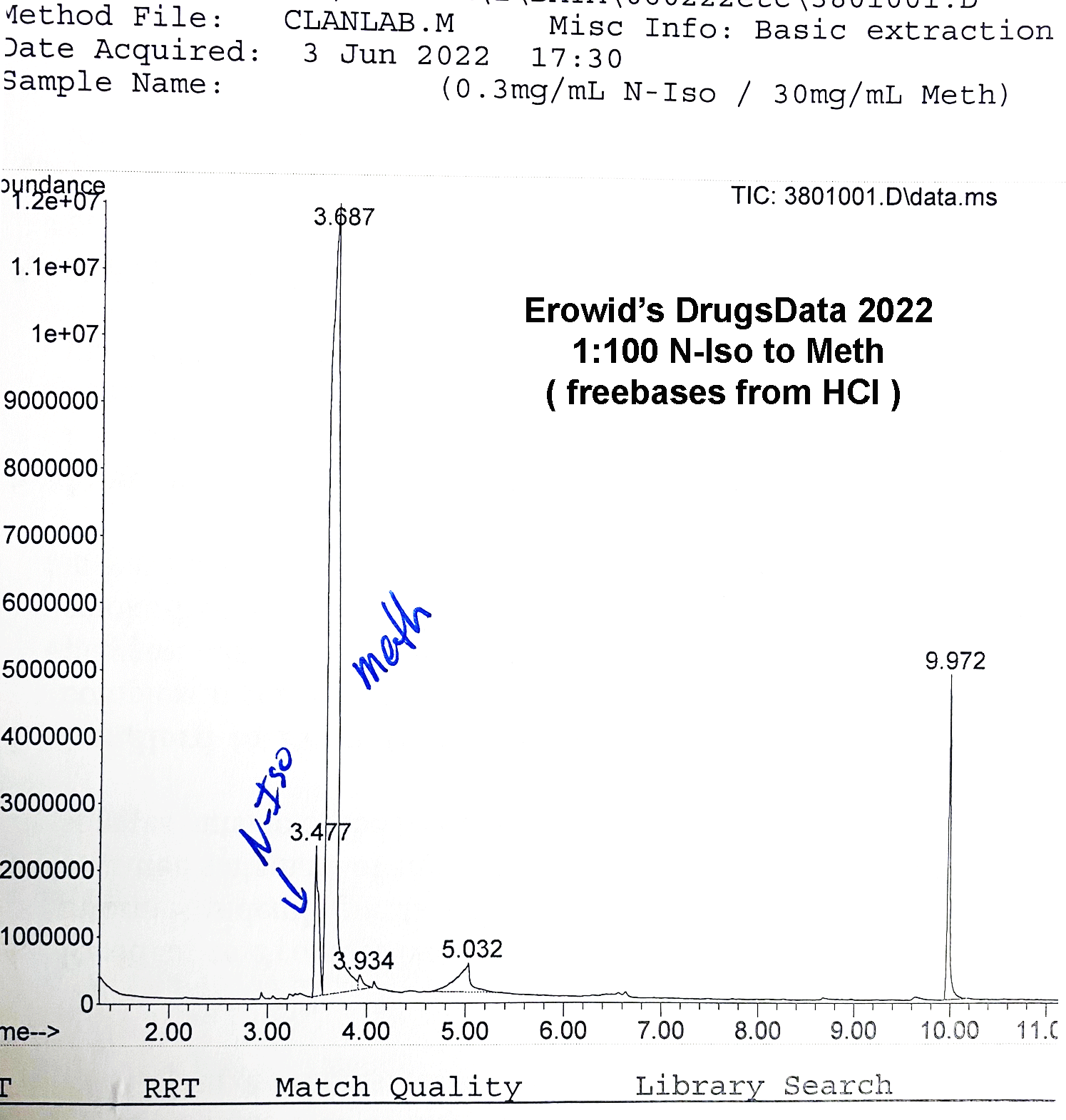
The GC output pictured above is from N-IPBA (“N-iso”) (1 part) mixed with meth (100 parts). The other main peak (9.972) is a calibration chemical. The slightly messy baseline to the right of the meth peak is related to the way that the salt versions (Meth HCl, for instance) of the two drugs differ in their elution times in the GC column. This example graph is after several tests in a row using different ratios of meth and isopropylbenzylamine. It is common when running methamphetamine salts to end up with a little right-side, baseline noise after the sharp freebase meth peak.
Methamphetamine and isopropylbenzylamine do elute at similar times, but using our procedure, they are clearly distinct. Note in the GC image the sharp valley between the N-iso and meth peaks: N-iso at 3.4777 minutes and meth at 3.687 minutes in this run.
And they always have clearly distinct Mass Spectra (MS), so they simply don’t get confused at our lab. If a lab were running a different column and procedure that isn’t targeted for doing work on methamphetamine and related drugs, it’s easy to imagine other procedures and rigs where an analytical chemist could confuse one with the other.
As of August 2022, DrugsData’s lab has found isopropylbenzylamine in eight samples total, ever, and two DEA-tested samples are republished in our database:
https://www.drugsdata.org/results.php?search_field=substance&s=Isopropylbenzylamine
It is Erowid’s view that most negative effects from meth use are a result of lack of sleep combined with irregular water and food consumption. People mistakenly attribute differences in experience from time to time to differences in impurities in the drugs, instead of other factors such as diet, mood, context, electrolyte levels, and physical rest.
It’s certainly possible that a sample analyzed in DrugsData’s lab in the future could contain both methamphetamine and isopropylbenzylamine. We feel certain that for such a sample, lab results would clearly show this to be the case.
—earth, Sylvia, Fire, Roi