
#150 3-TE
3-THIOESCALINE; 4-ETHOXY-5-METHOXY-3-METHYLTHIOPHENETHYLAMINE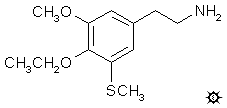
|
| [3D .mol structure] |
To a solution of 4.4 g 4-ethoxy-5-methoxy-3-(methylthio)benzaldehyde in 75 mL nitromethane, there was added 0.5 g anhydrous ammonium acetate and the mixture was heated on the steam bath for 80 min. Care must be taken in the length of time, and there must be frequent TLC montoring, as there is a rapid scrudge buildup (see under 3-TSB for a discussion of scrudge). The reaction mixture was stripped of nitromethane under vacuum, and the residual deep-yellow oil was dissolved in 20 mL of boiling MeOH. This was decanted from a small amount of insoluble matter and, upon cooling, deposited bright yellow crystals of 4-ethoxy-5-methoxy-3-methylthio-beta-nitrostyrene. This was removed by filtration and, after washing with cold MeOH and air drying, weighed 2.4 g. The mp was ambiguous. The above crude material melted at 92-93 °C, which is probably too high! Earlier samples which melted in the low 80's appeared to have a mp, after repeated recrystallization from MeOH, of 87-88 °C. This latter was the property of the analytical sample. Anal. (C12H15NO4S) C,H. The mp of the TLC low-moving component is always quite high, and might have been a factor in the assignment of this physical property.
AH was prepared in the usual manner from a suspension of 2.0 g LAH in 75 mL anhydrous THF, cooled to 0 °C, well stirred in an inert atmosphere of He, and treated with 1.33 mL of 100% H2SO4 added dropwise. There was added, dropwise and over the course of 10 min, a solution of 2.4 g 4-ethoxy-5-methoxy-3-methylthio-beta-nitrostyrene in 15 mL anhydrous THF. The reaction was exothermic, and was heated on the steam bath at reflux for an additional 10 min. After cooling again, there was added enough IPA to decompose the excess hydride and sufficient 10% NaOH to convert the aluminum oxide solids to a white, easily filterable mass. This was filtered, the filter cake washed with additional IPA, the filtrate and washes combined, and the solvent removed under vacuum. This was dissolved in 100 mL of dilute H2SO4 which was washed with 2x50 mL CH2Cl2. The aqueous phase was made basic with NaOH, extracted with 2x50 mL CH2Cl2, and the extracts pooled and the solvent removed under vacuum to yield a residue of a colorless oil. This distilled at 118-122 °C at 0.4 mm/Hg producing 1.9 g of a colorless oil. This was dissolved in 10 mL IPA, neutralized with 30 drops of concentrated HCl and, with good stirring, diluted with 20 mL anhydrous Et2O. The product 4-ethoxy-5-methoxy-3-methylthiophenethylamine hydrochloride (3-TE) was removed by filtration, washed with Et2O, and air dried to provide a white solid that weighed 1.0 g and melted at about 180 °C. Anal. (C12H20ClNO2S) C,H.
DOSAGE: 60 - 80 mg.
DURATION: 8 - 12 h.
QUALITATIVE COMMENTS: (with 60 mg) There may well be time slowing. I noticed that the voices on the radio seemed to be of a deeper pitch. And with music there is a most easy flight of fantasy. I tried to keep a logical conversation going on the telephone, but I am pretty sure there were problems. I found myself down sooner than I would have liked.
(with 70 mg) I found myself in a good, rich place, and thoroughly enjoyed my introspection. I didn't want to talk and interact, and that seemed just fine with everyone else. Several of the others seemed restless, but I lay back and let them do their thing. My appetite was fine towards the end, and I might have actually overeaten. I was able to drive home that evening, but there seemed to be some slight residual something after waking in the morning. I would certainly repeat without hesitation.
(with 80 mg) Art interpretation and imagery with music are remarkable. This material touches on the psychedelic--rather than just being stoned. The body is higher than the mind, but where the mind is makes it all OK. It's worth the cost. My getting to sleep was easy that evening, but sleep was not too restful and there was something strange about it.
EXTENSIONS AND COMMENTARY: There is a good lesson to be learned in the attempts to predict the potency of 3-TE before it was actually explored. All pharmacological prediction follows pretty much a single mechanism. Find things that are close in some way, and arrange them in a manner that allows comparison. A relates to B in this way, and A relates to C in that way, and since D incorporates both this and that of each, it will probably be such-and-such. The Roman square.
Here is the square with the horizontal arrow adding a sulfur in the 3-position and the vertical arrow adding an ethyl group in place of a methyl group at the 4-position:
Mescaline x 3.5 3-TM 200-400 mg ------------------------> 60-100 mg | | | x 6 | V V Escaline 3-TE "x20" 40-60 mg ------------------------> = 10-20 mg
and one would predict a potency of some 20x that of mescaline, or something in the range of 15 mg.
Here is an equally likely square, based on the horizontal arrow relocating a sulfur from the 4-position to the 3-position, and the vertical arrow again adding an ethyl group in place of a methyl group in the 4-position:
Thiomescaline x 0.3 3-Thiomescaline 20-30 mg ------------------------> 60-100 mg | | | x 1 | V V Thioescaline 3-TE "x0.3" 20-30 mg ------------------------> = 60-100 mg
and one would predict a potency of some one third of that of thiomescaline, or something in the range of 80 milligrams.
This latter square gave a prediction that was very close to the observed potency, but it would be careless, and probably wrong, to assume that the latter relationships had any more significance than the former ones. As one accumulates the potencies of many compounds it is tempting to draw complex relationships such as these, and to be seduced into believing that they must explain things. And, especially, beware the multivariable power of the computer which can explore monstrous numbers of variables at breakneck speeds, and spew forth fantastic correlations with marvelous ease.
But nothing can ever substitute for the simple art of tasting something new.
| [ |
[Main Index] | [Forward |

