
#8 ARIADNE
4C-DOM; BL-3912; DIMOXAMINE; 1-(2,5-DIMETHOXY-4-METHYLPHENYL)-2-AMINOBUTANE; 2,5-DIMETHOXY-a-ETHYL-4-METHYLPHENETHYLAMINE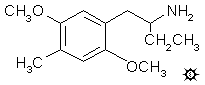
|
| [3D .mol structure] |
A suspension of 12.5 g LAH in 600 mL anhydrous THF was stirred magnetically, and brought up to a reflux. To this there was added, dropwise, 15.0 g 1-(2,5-dimethoxy-4-methylphenyl)-2-nitro-1-butene dissolved in 150 mL THF. Refluxing was continued for 15 h and, after cooling, the excess hydride was decomposed by the addition of 12.5 mL H2O. The inorganic salts were made loose and granular by the addition of 12.5 mL 15% NaOH followed by an additional 37.5 mL H2O. These solids were removed by filtration, and the filter cake was washed with THF. The combined filtrate and washings were stripped of solvent under vacuum. The residue was dissolved in anhydrous Et2O, and treated with hydrogen chloride gas, yielding 1-(2,5-dimethoxy-4-methylphenyl)-2-aminobutane hydrochloride (ARIADNE) as white crystals which, after recrystallization from IPA, weighed 11.4 g and had a mp of 232.5-234.5 °C. Anal. (C13H22ClNO2) C,H,N,Cl. The racemic mixture was resolved into its optical isomers by the formation of salts with (+)-2beta-nitrotartranilic acid (to give the "S" isomer) or with (+)-2beta-chlorotartranilic acid (to give the "R" isomer). The "R" isomer can also be prepared by the reductive amination of 1-(2,5-dimethoxy-4-methylphenyl)-2-butanone (from the above nitrostyrene and elemental iron) with (+)-a-methyl benzylamine followed by the hydrogenolysis of the benzyl group.
DOSAGE: as psychedelic, unknown.
DURATION: short.
QUALITATIVE COMMENTS: (with 12 mg) I believe that my mood has distinctly improved, and my sleep that evening was excellent. This is physically benign.
(with 32 mg) There was some sort of threshold that lasted for a couple of hours.
(with 25 mg of the "R" isomer) There is the alert of a psychedelic, with none of the rest of the package. Perhaps a bit of paranoia. And by the fifth hour everything is largely gone.
EXTENSIONS AND COMMENTARY: How does one discover a new drug for a malady that does not exist in experimental animals? Drugs that interfere with sleep, or with appetite, or with some infecting bacterium, are naturals for animal screening, in that animals sleep, eat, and can be easily infected. But there are lots of syndromes that involve a state of mind, and these are uniquely human. Many of the psychopharmacological anti-this or anti-that agents address ailments such as anxiety, psychosis, paranoia, or depression, which are only known in man. So how does one discover a new drug in areas such as these? If one has in hand a drug that is known to be effective in one of these human ailments, an animal assay can be set up to give some measurable response to that specific drug, or a biochemical property can be rationalized as being related to a mechanism of action. And with the known drug as a calibration, and restricting your search to structurally related compounds, you can find structural relatives that give the same responses.
But how does one find a new class? One way is to kind of stumble into it as a side-line of human experimentation with new psychedelics. But it is really difficult to pick up the clues as to what will be a good anti-depressant if you are not depressed. This compound, to which I had given the name of ARIADNE as the first of my ten "classic ladies" (I'll say more about them later), was not really a stimulant of any kind, certainly it was not a psychedelic, and yet there was something there. It had been explored rather extensively as a potential psychotherapeutic ally by a friend of mine. He said that there seemed to be some value in a few of his patients who had some underlying depression, but not much of anything with the others. So, I decided to call it an anti-depressant. I had mentioned some of this history one time when I was giving an address at a conference on the East Coast, and my host (who happened to be the research director at a large pharmaceutical house) asked if I would send him a sample. His company did many animal tests, one of which showed that it was not hallucinogenic (a cat whose tail erected dramatically with DOM did nothing with ARIADNE) and another that showed re-motivation (some old maze-running monkeys who had decided not to run any more mazes changed their minds with ARIADNE).
So patents were obtained for the "R" isomer, the more effective isomer, covering its use for such things as the restoring of motivation in senile geriatric patients. And a tradename of Dimoxamine was assigned it, despite several voices that held out for Ariadnamine. But it didn't have what was needed to make it all the way to the commercial market
Many, many analogues of ARIADNE have been made, and for a variety of reasons. In the industrial world there is research backup carried out, not only for the discovery of new things, but also for patent protection of old things. Several dozen analogues of ARIADNE have been made and pharmacologically evaluated, and some of them have been put into the published literature. The major points of variation have been two: keep the 4-position methyl group intact, and make the variations on the alpha-carbon (propyl, butyl, dimethyl, phenyl, benzyl, phenethyl, etc.--an extensive etc.) or: keep the alpha-position ethyl group intact and make the variations on the 4-position (chloro, iodo, methylthio, carboxy, etc.--again, an extensive etc.).
Some of these analogues I had made, and sent in for animal screening. The high potency of DOB suggested the bromo-counterpart of ARIADNE. The making of this entailed the proteo counterpart, 1-(2,5-dimethoxyphenyl)-2-aminobutane. Reaction of 2,5-dimethoxybenzaldehyde with nitropropane in benzene in a Dean Stark apparatus with cyclohexylamine as a catalyst produced 1-(2,5-dimethoxyphenyl)-2-nitrobutene, which crystallized as orange crystals from MeOH with a mp of 47-47.5 °C. Anal. (C12H15NO4) C,H,N. This was reduced to the amine 1-(2,5-dimethoxyphenyl)-2-aminobutane with LAH in ether, and this gave a hydrochloride salt with a mp of 172-174 °C after recrystallization from acetonitrile. The free base of this compound was brominated in acetic acid to give 1-(2,5-dimethoxy-4-bromophenyl)-2-aminobutane which yielded a white hydrochloride salt with a mp of 204-206 °C following recrystallization from IPA. The isomeric non-brominated analogue, 1-(3,4-dimethoxyphenyl)-2-aminobutane was made and explored by the Chemical Warfare group at Edgewood Arsenal; its code number is EA-1322.
Several of the alpha-ethyl analogues of ARIADNE were N,N-dialkylated, and were target compounds for halogenation with radio-iodine or radio-fluorine, for evaluation as potential brain blood-flow indicators. In these studies. all examples followed a common flow diagram. The reaction of the appropriate benzaldehyde and nitropropane, using N,N-dimethylethylenediamine as a catalyst and following recrystallization from MeOH, gave the corresponding 1-aromatic-2-nitro-1-butene (the nitrostyrene) which, by reduction with elemental iron, gave the corresponding 2-butanone (which was distilled at about 0.3 mm/Hg). This led, by reductive amination with dimethylamine hydrochloride and sodium cyanoborohydride, to the corresponding N,N-dimethyl product which was distilled at about 0.3 mm/Hg and which, in no case, either formed a solid HCl salt or reacted with carbon dioxide from the air. From 2,4-dimethoxybenzaldehyde, the nitrostyrene appeared as yellow crystals, the ketone as a white oil, and the product N,N-dimethyl-1-(2,4-dimethoxyphenyl)-2-aminobutane as a white oil. From 2,5-dimethoxybenzaldehyde, the nitrostyrene formed bright yellow crystal, the ketone was an off-white oil, and the product N,N-dimethyl-1-(2,5-dimethoxyphenyl)-2-aminobutane was a white oil. From 3,5-dimethoxybenzaldehyde, the nitrostyrene formed pale yellow crystals that discolored on exposure to the light, the ketone was an off-white clear oil, and the product N,N-dimethyl-1-(3,5-dimethoxyphenyl)-2-aminobutane was a white oil. From 2,6-dimethoxybenzaldehyde, the nitrostyrene was obtained as orange crystals, and was not pursued further.
A number of ARIADNE analogues have been made, or at least started, purely to serve as probes into whatever new areas of psychopharmacological activity might be uncovered. One of these is a HOT compound, and one is a TOM compound, and a couple of them are the pseudo (or near-pseudo) orientations. The HOT analogue was made from the nitrostyrene precursor to ARIADNE itself, reduced not with LAH or AH (which would give the primary amine), but rather with sodium borohydride and borane dimethylsulfide. The product, 1-(2,5-dimethoxy-4-methylphenyl)-N-hydroxy-2-aminobutane hydrochloride, was a white crystalline material. The 5-TOM analogue got as far as the nitrostyrene. This was made from 2-methoxy-4-methyl-5-(methylthio)benzaldehyde (see under the 5-TOM recipe for its preparation) and nitropropane in acetic acid, and gave bright yellow crystals. The true pseudo-analogue is the 2,4,6-trimethoxy material based on TMA-6, which is the "real" pseudo-TMA-2. The nitrostyrene from 2,4,6-trimethoxybenzaldehyde and nitropropane crystallized from MeOH/CH3CN as fine yellow crystals, and this was reduced with AH in cold THF to 1-(2,4,6-trimethoxyphenyl)-2-aminobutane which was a bright, white powder.
And the near-pseudo analogue?
First, what is near-pseudo? I have explained already that the "normal" world of substitution patterns is the 2,4,5. Everyone knows that that is the most potent pattern. But, the 2,4,6 is in many ways equipotent, and has been named the pseudo-stuff. The "real," or "true" pseudo-stuff. So what is the "near" pseudo-stuff? I am willing to bet that the rather easily obtained 2,3,6-trisubstitution pattern, and the much more difficult to obtain 2,3,5-substitution pattern, will produce treasures every bit as unexpected and remarkable as either the 2,4,5- or the 2,4,6- counterparts. These are neither "real" nor "pseudo," but something else, and I will find a name for them when the time comes, something weird from the Greek alphabet. And this will double again the range of possible exploration. The TMA-5 analogue mentioned came from 2,3,6-trimethoxybenzaldehyde and nitropropane using cyclohexylamine as a catalyst (yellow-orange solids) which was reduced to the amine with AH. This hydrochloride salt is an air-stable white powder. All of these materials remain unexplored.
Somewhere in the wealth of compounds implicit in the many structural variables possible (the normal versus the pseudo versus the near-pseudo patterns, coupled with the wide variety of promising substituents that can be placed on the 4-position, together with the availability of the the unexplored members of the Ten Classic Ladies harem), it would seem inescapable that interesting compounds will emerge.
Just what is this all about the ten "Classic Ladies?" In the chemical struc-ture of DOM, there is a total of nineteen hydrogen atoms. Some of these are indis-tinguishable from others, such as the three hydrogen atoms on a methyl group. But there are exactly ten "types" of hydrogen atoms present. And, not having much, if any, intuition as to just why DOM was so powerful a psychedelic, I decided to systematically replace each of the ten unique hydrogens, one at a time of course, with a methyl group. And I planned to give the resulting materials the names of famous ladies, alphabetically, as you walk around the molecule.
ARIADNE was the first of these, the methyl for a hydrogen atom on the methyl group of the amphetamine chain. It was Ariadne who gave the long piece of thread to Theseus to guide him through the mazes of the Labyrinth so he could escape after killing the Minotaur. The record is fuzzy as to whether, after the successful killing, she went with him, or let him go on alone. A methyl group on the nitrogen atom produced BEATRICE. There is the legendary Beatrijs of the Dutch religious literature of the 14th century, and there is the Beatrice from Beatrice and Benedict (of Berlioz fame). But the one I had in mind was the lady from Florence whom Dante immortalized in the Divina Commedia, and she is entered under her own name in this footnote. Replacing the alpha-hydrogen of DOM with a methyl group would give the phentermine analogue which is named CHARMIAN. You may be thinking of Cleopatra's favorite attendant, but I was thinking of the sweet wife of a very dear friend of mine, a lady who has been in a state of gentle schizophrenia for some forty years now. The MDA analogue of CHARMIAN has been described in this foornote under the code name of MDPH. CHARMIAN, herself, has been synthesized and is of very much reduced potency in animals, as compared to DOM. It has not been tried in man as far as I know.
The two beta-hydrogen atoms of DOM are distinct in that, upon being replaced with methyl groups, one would produce a threo-isomer, and the other an erythro-isomer. I have named them DAPHNE (who escaped from Apollo by becoming a laurel tree which was, incidentally, named for her) and ELVIRA (who might not be too well known classically, but whose name has been attached to Mozart's 21st piano concerto as its slow movement was used as theme music for the movie Elvira Madigan). I don't know if either of this pair has been made--I started and got as far as the cis-trans mixture of adducts betweeen nitroethane and 2,5-dimethoxy-4-methylacetophenone. Whoever finally makes them gets to assign the names. I had made and tested the corresponding homologues of DMMDA that correspond to these two ladies.
And there are five positions (2,3,4,5 and 6) around the aromatic ring, each of which either carries a hydrogen atom or a methyl group that has a hydrogen atom. There is the 2-methoxy group which can become a 2-ethoxy group to produce a compound called FLORENCE. Her name is the English translation of the Italian Firenze, a city that, although having a female name, has always seemed thoroughly masculine to me. There is the 3-hydrogen atom which can become a 3-methyl group to produce a compound called GANESHA. This is a fine elephant-headed Indian God who is the symbol of worldly wisdom and also has been seen as the creator of obstacles. Here I really blew it; the Classic Lady turned out to be a Classic Gentle-man; not even the name is feminine. There is the 4-methyl group which can become a 4-ethyl group to produce a compound called HECATE who presided over magic arts and spells. There is the 5-methoxy group which can become a 5-ethoxy group to produce a compound called IRIS, who is the Goddess of the rainbow. And there is the 6-hydrogen atom which can become a 6-methyl group to produce a compound called JUNO, who is pretty much a lady's lady, or should I say a woman's woman.
GANESHA, 2,5-dimethoxy-3,4-dimethylamphetamine has been made, and has proven to be an extraordinary starting point for a large series of potent phenethylamines and amphetamines which are described in this book. HECATE was given a synonym early in this process, and is now known as DOET (2,5-dimethoxy-4-ethylamphetamine). IRIS has also been entered under her name, and the other ethoxy homologue, FLORENCE, would be easily made based on the preparation of the phenethylamine analogue, 2CD-2ETO. Perhaps it has already been made somehow, somewhere, as I have noted that I have claimed its citrate salt as a new compound in a British patent. And, finally, JUNO (3,6-dimethoxy-2,4-dimethylamphetamine) has been made (from 2,5-dimethoxy-m-xylene, which was reacted with POCl3 and N-methylformanilide to the benzaldehyde, mp 53-54 °C, and to the nitrostyrene with nitroethane, mp 73-74 °C from cyclohexane, and to the final amine hydrochloride with LAH in THF). Rather amazingly, I have had JUNO on the shelf for almost 14 years and have not yet gotten around to tasting it.
| [ |
[Main Index] | [Forward |

