Ayahuasca: alkaloids, plants & analogs
Section 1 :
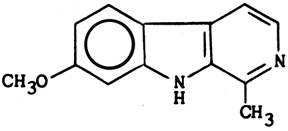
Structure & properties of Harmine
C13H12N2O
MW 212.25 Merck Index
MW 212.251 Southon & Buckingham 1989
MW 212.27 Sax & Lewis 1989 (entry #HAI500)
MW 212.3 Clarke's Second 1986
MW 212.55 CRC 1980-1981
C 73.56%, H 5.70%, N 13.20%, O 7.54%
Merck Index and Ott 1993
Free base: Slender orthorhombic prisms from methanol, mp 261° (dec.) Merck Index
mp 253° (beautiful white needles) Perrot & Raymond-Hamet 1927
mp 256° Manske 1965
mp 257° Long needles from dilute aqueous ammonia. Hasenfratz 1927
mp 257-258° Telezhenetskaya 1977
mp 258° Large needle shaped. Rozenfeld 1931
mp 258° Chatterjee & Ganguly 1968
mp 258-259° (synthetic) 260-261.5° (from yage) Elger 1928 (Both were believed contaminated with harmaline)
mp 260° (corr.- some decomposition) White shiny crystals. O'Connell & Lynn 1953
mp 261° (dec.) Prisms from methanol. Ott 1993 (Exptl.- 262°; greenish crystals from P. harmala.)
Crystallized directly from hot chloroform with concentration. Recrystallized from methanol (twice) to yield material mp 261.8-262.4°. mmp with synthetic was not depressed. Hochstein & Paradies 1957 page 5736
mp 261° (with decomposition or sublimation) Clarke's Second 1986
mp 263-264° (from MeOH) Bernauer 1964. (colorless needles from Ether) Elger 1928
mp 264-265° (257-259°) Southon & Buckingham 1989
mp 266° Neuss 1964
mp 272-274° Rhombic crystals from alcohol. Prisms from methanol. CRC 1980-1981)
Sublimes in vacuum. Hochstein & Paradies 1957
Sublimes. CRC 1980-1981 and Merck Index.
Sublimes in vacuum yielding colorless needles. Ghosal & Mazumdar 1971
Insoluble in H2O [Note 1]
Soluble in alcohol, benzene, chloroform and pyridine.
Less water soluble than harmaline. Precipitates before it in fractional crystallization.
Hasenfratz 1927
Soluble in pyridine. CRC 1980-1981
Slightly soluble in water, alcohol, chloroform, ether.
Merck Index and Ott 1993
Soluble in alcohol, methanol, chloroform and acids.
Slightly soluble in water or alkalies. (Precipitated from acid solution by neutralizing with ammonia)
O'Connell & Lynn 1953
Soluble in chloroform, ether, methanol and dilute sulfuric acid
Drost-Karbowska et al. 1978
Soluble with difficulty in Chloroform. Soluble in dilute acids. Poethke et al. 1970
Soluble in Ether. Hashimoto & Kawanishi 1975
Slightly soluble in Water, Ethanol, Chlorofom, Ether. Clarke's 1986
K = 10-6.05 [Ed.: pK= log 1/K= 6.05]
Merck Index
pKa 7.6 (20o) Clarke's Second 1986
Aqueous solutions fluoresce blue. [Ed.: See more on harmine's fluorescence elsewhere here.] Hasenfratz 1927
Salts in solution fluoresce blue; after acidification with Sulfuric acid fluorescence is green. Elger 1928
Fluorescence:
- Marion 1952 p. 394 cited Bertrand 1945
- Excitation maximum 300, 365 nm
Fluorescent maximum 400 nm
Optimal pH 1
Udenfriend 1962
IR:
- Neuss 1964
Shulgin & Shulgin 1997
IR and chromatographic behavior of isolated material is identical to synthetic material.
Synthetic was prepared by nitric acid oxidation of commercial harmaline using procedure of Iyer & Robinson 1934.
Hochstein & Paradies 1957 page 5736
MS:
- Drost-Karbowska et al. 1978 [m/e 212]
Holmstedt & Lindgren 1967.
Shoemaker et al. 1979
Shulgin & Shulgin 1997
- NMR (graphic): Chatterjee & Ganguly 1968
- UV:UV λmax (in methanol): ε241 = 40,900, ε301 = 16,100, ε336 = 4,930. Neuss 1964
UV λmax (methanol): 241, 301, 336 nm (log ε 4.61, 4.21, 3.69) Merck Index
UV λmax (methanol): 209, 240, 252sh, 302, 325, 338 nm. Drost-Karbowska et al. 1978
UVλmax (Ethanol) 239, 299, 324 & 326 nm. Chatterjee & Ganguly 1968
See also Doig et al. 1952
and Cheng & Mitchelson 1997
UVmax 300 mm
UVmin 272 mm
O'Connell & Lynn 1953
X-ray powder data: Neuss 1964
Chromatography:
- GLC:
Shoemaker et al. 1979
Liquid [Note 2]: Eluted from alumina with Chloroform.
Ghosal & Mazumdar 1971
tlc:
Solvent system Rf
Methyl alcohol-Chloroform-Acetic acid (75:25:15) 0.73
Ghosal et al. 1971 (on Silica Gel G chromatoplates)
Chloroform-Formamide 0.64
Hochstein & Paradies 1957 citing Hochstein et al. 1955
Acetone-Ethanol (85:15)
0.64 Bernauer 1964 (on Alumina)
On Silica Gel (Dipped or sprayed with Potassium hydroxide [0.1M] and dried)
System Rf
Methanol-conc. Ammonium hydroxide
(100:1.5) 0.63
Cyclohexane-Toluene-Diethylamine
(75:15:10) 0.00
Chloroform-Methanol
(90:10) 0.22
TLC reagent: Acidified Iodoplatinate
Clarke's Second 1986
Detection using Fluorescence in Liquid chromatography:
(Stationary phase: alkyl-modified silica gel/ Mobile phase Methanol-Water-Formic acid (166:34:1) adjusted to pH 8.5 with triethylamine.)
Excitation: 304
Emission: 355
Popl et al. 1990
Electrophoresis (relative displacement values):
Relative displacement values; on Whatman No. 1 paper, 18X46 cm, at 8 v/cm for 3 hours in the presence of Universal buffer:
| pH | 2.3 | 4.3 | 6.4 | 8.2 | 10.5 | 11.4 | ||||||
| Harmine | 41 | 31 | 26 | 0 | 0 | 0 |
Marini-Bettòlo & Coch Frugoni 1958
(Using LKB apparatus of Volmet & Svensson) See a later section here for more details
Electrophoretic separation was also reported by Cheng & Mitchelson 1997 using high-performance capillary electrophoresis (micellar electrokinetic chromatography).
Chromophores of Harmine:
Easily visualized under UV without any chromophoretic agent. (Color depends on pH)
TLC reagent: Acidified Iodoplatinate
Clarke's 1986
On silica gel:
Dragendorff's: Orange
Ehrlich's: Negative
a-Nitroso-ß-naphtholnitrous acid: Dull violet
Ghosal et al. 1971.
Using pure material:
Mandelin's reagent [Note 3]: Blue becoming Green.
0.25 mg sensitivity.
Meckes reagent [Note 4]: Green becoming Yellow.
0.05 mg sensitivity.
Farmilo & Genest 1961
PDAB: Red
Mandelin's Test: Blue changing to Green
Marquis test: Orange
Clarke's Second 1986
Concentrated Sulfuric acid: Green-Yellow
Concentrated Sulfuric acid with Potassium bichromate: Fleeting, streaked purple-blue
Nitric acid:
Green turning greenish-blue then blue-green becoming black. If heated in waterbath becoming blue-purple, evaporates to purple residue.
Vitali: (Using residue from Nitric acid test)
Upon addition of a potash solution, becomes beautiful orange color.
Perrot & Raymond-Hamet 1927
Assay (mostly from Usdin & Efron 1979:
DerMarderosian et al. 1968
and Clarke 1969
and Slotkin 1970
See also Clarke's Second edition, 1986 which includes TLC, GC, HPLC, UV (graphic), IR (graphic) & MS.
See also fluorescence, tlc, color reactions and UV absorption above
Isolations and analysis:
(See Deulofeu 1967 and Ott 1994 for a historical discussion and far more details.)
- Göbel 1841 (First isolated what he thought was a single alkaloid from Peganum harmala: he named it harmaline. It was eventually shown to be a mixture of harmine and harmaline.)
Fritzsche 1847 (Also from Peganum harmala)
Zerda Bayón 1915 [In 1905, isolated an amorphous preparation he called telepatina or telepathine]
Albarracín 1925 [Isolated yagéina and yajénina from material [mis]identified as Prestonia amazonica. Also published in Barriga Villalba 1925a and 1925b .]
Fischer Cardenas 1923 Isolated what he thought was a single base. [Also used name telepathine for the (impure) base he isolated]
Barriga Villalba 1925 The first to isolated a crystalline (but impure) product from Banisteriopsis caapi; he named it yajéine. It was most likely harmine. He also noted the presence of another base in the mother liquor, named it yagénine but included no constants for it. [He originally identified his material as Haemadictylon amazonicum (Prestonia amazonica) [Identity was based on Reinberg] but later stated that the material was Banisteriopsis caapi.
Clinquart 1926 and Michiels & Clinquart 1926 [Isolation of yagéina and yajénina from material identified as Prestonia amazonica. ]
Hasenfratz 1927 [From Peganum harmala seeds.]
Perrot & Raymond-Hamet 1927a and 1927b [The first to isolate pure material from Banisteriopsis caapi. Kept name telepathine. Reported equivalence of télépathine and yagéine.]
Lewin 1928 and 1929 [Reported isolation of Banisterine from Banisteria [sic] caapi and stated it was considered identical to harmine by chemists at E. Merck.]
Elger 1928 [Showed that harmine from Yage was identical with that isolated from Peganum harmala.]
Wolf & Rumpf 1928 [Workers from Merck who showed that harmine from Banisteriopsis was identical with that isolated from Peganum harmala.]
Brückl & Mussgnug 1929, Dalmer 1929, Elger 1928, Keller & Gottauf 1929 and Wolfes & Rumpf 1928 [Reported equivalence of telepatina, yagéina and banisterine]
Rozenfeld 1931 [From Peganum harmala roots]
Arispe 1938 [Reported the isolation of yagéina and yagénina from Banisteriopsis caapi.]
Chen & Chen 1939 and Williams 1931 [Showed that telepathine, yagéine and banisterine were identical to harmine. Isolated harmine from stems, leaves and roots of Banisteriopsis caapi]
O'Connell & Lynn 1953 [Isolated only harmine from Banisteriopsis inebrians.]
Mors & Zaltzman 1954 [From both Banisteriopsis caapi and Cabi paraensis]
Hochstein & Paradies 1957 [Thought to be from Banisteriopsis caapi; they assumed the identity as they actually analyzed a previously prepared ayahuasca brew. Said to be from Banisteria caapi [sic].]
Poisson 1965 [Isolated harmine from Banisteriopsis inebrians and mentioned the presence of small amounts of harmaline.]
Reinhard et al. 1968
Synthesis:
- Manske et al. 1927
Spath & Lederer 1930a
Spath & Lederer 1930b
Akaboro & Saito 1930
Hahn et al. 1934
Iyer & Robinson 1934 (from harmaline)
Hahn et al. 1938
Harvey & Robinson 1938
Cook et al. 1951
Shulgin & Shulgin 1997 (from harmaline)
Harmine hydrochloride:
- Usually yellow. "completely white" with two recrystallizations after pure base is obtained according to Hasenfratz.
Long needles (under a microscope) mp 257° (from dilute aqueous ammonia)
Hasenfratz 1927
White crystals mp 261° (dec.) (anhydrous- mp 319° - 12.5% Cl) O'Connell & Lynn 1953
Hydrochloride dihydrate: crystals, mp 262° (dec.) [mp 321° when anhydrous]
Merck Index and Ott 1993
mp 262° Rozenfeld 1931
mp 264° (dec.) Fine needles (from Ethanol by addition of Ether containing HCl) Wolfes & Rumpf 1928
Crystallized as hydrochloride from AcMe-MeOH. Ghosal & Mazumdar 1971
Soluble in water. Ott 1993
Soluble in 40 parts of water, freely soluble in hot water. Merck Index.
Insoluble in cold Sodium chloride solutions. Hasenfratz 1927
H2O solutions are Fluorescent Rozenfeld 1931
Aqueous solutions have blue fluorescence. Merck Index.
Crystalline material showed blue fluorescence. O'Connell & Lynn 1953
Fluoresces indigo blue in acid solutions and yellow-green in alkaline (transition within the interval pH 7.2-8.9) Marion 1952 p. 394 citing Izmailov & Shraiber 1938
Formed by the action of Sodium chloride on Harmine acetate. Hasenfratz 1927
MS and IR of harmine hydrochloride monohydrate: Shulgin & Shulgin 1997
Methiodide:
mp 306° (dec.) Chatterjee & Ganguly 1968
Picrate:
mp 249-250° (dec.)
Southon & Buckingham 1989
Acetate:
Water soluble. Readily converted to the hydrochloride by the action of Sodium chloride. Hasenfratz 1927
Nitrate & Perchlorate are slightly water soluble, flexible needles. Wolfes & Rumpf 1928
Notes #
- Insoluble is defined as when less than a gram of a compound is able to dissolve in a liter of liquid. Dennis McKenna analyzed a 60 ml sample of prepared ayahuasca and found 280 mg of harmine, 96 mg of d-leptaflorine, 25 mg of harmaline and 36 mg of DMT. Clearly sparingly or slightly soluble is more accurate.
- Liquid Chromatography: Hochstein & Paradies 1957; obtained a mixture of Harmine, Tetrahydroharmine and Harmaline from previously brewed Ayahuasca. They found that the entire mixture sublimed completely at 185° (0.1 mm). They separated it using Florisil, an activated Magnesium silicate. Chromatographing 0.5 grams of mixed alkaloids over 30 grams of Florisil. Elution was with Chloroform followed by stepwise increments of Chloroform and methanol, ending with pure Methanol. Harmine came off first, Tetrahydroharmine second and Harmaline last. Ghosal & Mazumdar 1971 similarly reported they had eluted Harmine from alumina with Chloroform and eluted Harmaline with Methanol.
- Mandelin's reagent: "A microdrop of the test solution is placed on a plate of opal glass and a similar drop of a 0.5% solution of aqueous Ammonium vanadate is added. After evaporation, [a] microdrop of concentrated sulfuric acid is added and the color changes noted."
- Meckes reagent: "A microdrop of the test solution is placed on a plate of opal glass and a similar drop of a 0.5% solution of aqueous Selenious acid is added. After evaporation, [a] microdrop of concentrated sulfuric acid is added and the color changes noted."

