|
Sulfurous Samadhi: Table of Contents Intro Chemistry History Stolaroff Survey Hardison's Survey User Survey Pharmacology Legal Conclusion Appendices: User Quotes: 2C-T-2 2C-T-7 Comparisons Synthesis: Product Lit: |
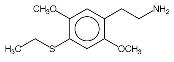
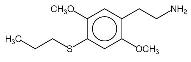
Chemistry and Physical Properties by Murple, Feb 6, 2001 2C-T-2 and 2C-T-7 are two closely related chemicals in a series of phenethylamine compounds created by Alexander Shulgin. The 2C prefix derives from the fact that these compounds are the two carbon phenethylamine homologues of previously made three carbon amphetamines, having the alpha-methyl group removed. Shulgin named most of the 2C compounds by adding the last letter of the amphetamine prototype's name, for example 2C-B from DOB and 2C-I from DOI. The 2C-T series is based on the three carbon ALEPH series (described in detail in Shulgin's book, PiHKAL), and the T comes from the fact that the original name for ALEPH-1 was DOT (T standing for "thio", from it's sulfur atom). There are a whole series of 2C-T compounds which have been made or theorized, and the numbering of the series was assigned in the order the chemicals were thought up, rather than due to any structural properties.
So far, the only syntheses which have been published are Shulgin's methods as described in PIHKAL. Shulgin's recipes for 2C-T-2 and 2C-T-7 have been included as appendices to this paper. Physical Properties2C-T-2's full name is 2,5-dimethoxy-4-ethylthiophenthylamine, and 2C-T-7's is 2,5-dimethoxy-4-(n)-propylthiophenethylamine. Both chemicals generally come in the form of a hydrochloride salt, which are white crystalline powders. As free bases, both chemicals are a clear white oil. 2C-T-2 has the molecular formula C12H19NO2S and a molecular weight of 241.3476. 2C-T-7, has the molecular formula C13H21NO2S and its molecular weight is 255.3744. 2C-T-2 has a boiling point of 120-130° Celsius (at 300 microns). 2C-T-7 has a boiling point of 140-150° Celsius at 251 microns. Both chemicals react to Marquis reagent, the principal ingredient in ecstasy testing kits, by producing an orange-red color reaction (similar to the color of uncooked salmon). 2C-T-2 produces a slightly more orange color, and 2C-T-7 a slightly more red color, but the difference is minute. Regarding the stability of these chemicals, in 2000, Shulgin re-examined the original discovery samples of 2C-T-7 and the closely related 2C-T-4, to make sure that the propyl and isopropyl groups, respectively, had not been compromised. These samples were at the time well into their second decade. Both samples were still white, and the spectra showed clean when checked by mass spectroscopy. When asked about the possibility of long term storage of 2C-T-2 and 2C-T-7, he said "I feel that the solids are stable with time." It is probably advisable to store the chemicals in air-tight, light-proof containers (such as amber glass vials with good caps) in a cool, dry location. Since these chemicals have become more widely available, some people have been dissolving them in solvents to allow for measuring doses out by liquid volume. The two most popular solvents for this purpose have been water and ethyl alcohol. There have been mixed reports as to the solubility of 2C-T-2 and 2C-T-7. Some have reported that when making solutions using water, with time, the drugs recrystallize and settle to the bottom. Reports of both unexpectedly strong and weak reactions raise questions about just how soluble these chemicals are. One factor which needs to be accounted for as well is that the solvents being used are not pure. Although advocates of this measurement technique recommend using distilled water, it is possible that people are also using plain bottled water and even unfiltered tap water. Some people use high proof liquor such as vodka or grain alcohol as a solvent. This offers the advantage of protecting against biological contaminants, and appears to offer somewhat better solubility. Regardless of which solvent is used, the impurities present in both liquor and water could conceivably alter the ability of the phenethylamines to enter and remain in solution, and could potentially react with them chemically. Another factor which could alter the ability of the chemicals to remain in solution is the temperature at which the solution is stored. Solutions which are stable at room temperature may separate out if stored in the refrigerator. The long term molecular stability of 2C-T-2 and 2C-T-7 in solution has never been explored, and there is the possibility that they may be less stable when stored in solution versus being stored in pure form. Related ChemicalsShulgin has thought up and named twenty-four compounds in the 2C-T series of phenethylamines. Nine of these have been synthesized and evaluated up to active doses, two have been synthesized but have not had their activities discovered, and the rest have never been made. There is also a twenty-fifth chemical in this series which not only was never made, but never officially assigned a 2C-T-X code name.
After all this, Shulgin decided that while the 2C-T family of drugs was pharmacologically interesting, it no longer seemed too challenging from a creative chemistry perspective. Realizing that others could pick up with them where he left off, using the knowledge in PIHKAL, he abandoned further research with the 2C-T series. Information gleaned from this research went on to bear other interesting fruit, however. Seduced by the unexpected enhancement of activity achieved by substituting sulfur for oxygen, Shulgin turned his attention back to mescaline and its derivatives escaline and meta-escaline. He spent several months synthesizing sulfur analogues of these, a process culminating in the creation and evaluation of all five possible compounds. The results were positive. 3-Thioescaline (3-TE) was found active in the 60 to 80 mg range, and 4-TE in the 20 to 30 mg range. 3-Thio-meta-escaline (3-TME) as well as 4-TME were both found active in the range of 60 to 100 mg. 5-TME has been run up to 200 mg, and if it is active, the dosage must be higher than this. Including an ethoxy group into the mescaline molecule and then inserting a sulfur atom was found to be able to increase the potency up to twenty times that of mescaline (as in the case of 4-TE). Further offspring of this line of research can be found in PIHKAL, including thioproscaline (which exhibited some unpleasant effects) and 4-thiobuscaline (which appears to be primarily a euphoriant). Shulgin then went on to explore this ethoxy/thio substitution in the tryptamine world, documenting some of his finds in TIHKAL. Regarding further explorations of the 2C-T series: if anyone has continued this research as Shulgin hoped, the results have not been made publically available. This field is ripe for picking.
|


|
|
|
|
|
|