
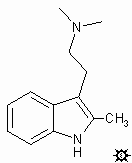
#34. 2-ME-DMT
2,N,N-TMT; TRYPTAMINE, 2,N,N-TRIMETHYL; INDOLE, 3-[2-(DIMETHYLAMINO)ETHYL]-2-METHYL; 2,N,N-TRIMETHYLTRYPTAMINE; 3-[2-(DIMETHYLAMINO)ETHYL]-2-METHYLINDOLE; DESMETHOXY-INDAPEX
|
| [3D .mol structure] |
A stirred solution of 3.8 g 2,N,N-trimethylindoleglyoxamide in 70 mL dry toluene was placed under a nitrogen pad and cooled with an external ice bath. There was then added 25 mL of a 60% solution of sodium bis(2-methoxyethoxy)aluminumhydride in toluene (Red-Al). The stirring was continued at 0 °C from 30 min, then brought to room temperature for an additional 2 h. After cooling again, the excess hydride was destroyed by the dropwise addition of IPA, and (when the gas evolution had ceased) H2O was added with caution. The aluminum salts were removed by filtration, and washed with isopropyl acetate. The filtrate and washes were combined, washed with H2O, then extracted with dilute HCl. After washing the aqueous phase with CH2Cl2, it was made basic with 20% aqueous KOH, and extracted with CH2Cl2. The pooled extracts were washed with H2O, dried over anhydrous Na2SO4, and the solvent removed under vacuum. The residue was dissolved in a small amount of MeOH and brought to a neutral pH with the careful addition of fumaric acid in MeOH. Removal of the solvent under vacuum gave a white crystalline residue which was washed with isopropyl acetate, and recrystallized from a MeOH / isopropyl acetate mixture. There was thus obtained 1.8 g 2,N,N-trimethyltryptamine fumerate (2,N,N-TMT) as colorless crystals with mp 205-208 °C.
The compound has also been synthesized from 3-methylindoleacetic acid via the ethyl ester, reduction with sodium and alcohol to the ethanol, to the ethyl bromide with PBr3 in Et2O, to the product (2-Me-DMT) with dimethylamine. The reported mp of the free base is 97-98 °C.
DOSAGE : 50 - 100 mg, orally
DURATION : 4 - 6 hrs
QUALITATIVE COMMENTS : (with 50 mg, orally) "There was tingling everywhere but it faded after about three hours. Nothing else."
(with 75 mg, orally) "Very mild stomach rumbling during the first hour, with no other effects until the 65 minute point. Then there was the onset of as very mild relaxed feeling followed by intermittent skin alerting, especially on the head and neck. No visuals. Sexual activity at 90 minutes showed marked enhancement of both the pre-climactic and orgasmic phase, which was confirmed by repeat activity at 120 and 180 minutes. When I switched on TV to a familiar news announcer, I thought that he had a cold because his voice sounded lower than normal, and throaty. Later I picked up a phone to call a friend and both the dial tone and the touch-tones sounded very unusual. Music at this point sounded normal, but I am sure that some tonal perception was altered by this drug. The effects seemed almost gone by 4 hours and were undetectable by 5 hours. Appetite seemed unaffected throughout, and dinner at the 5-hour point was very good. No GI problems occurred, and there were no after effects the next day."
(with 90 mg, orally) "The entire body was becoming activated (in a good way) but not much going on in the head. I am mentally clear but with the entire touch system a bit more activated than I would choose. This peaked at 3 hours, and was gone in another 3 hours. Everything is tactile."
(with 120 mg, orally) "There is as much to be said for what didn't happen as for what did. No visual changes. No cloudiness of the thought processes. No motor impairment what-so-ever. There was some down-shifting of music, with some distortion, which was overall more annoying than interesting. But I am glad I am alone because I cannot wear clothing. Anything touching the skin makes all my hair stand on end. The erection of my nipples is almost painful. Exploring sexual stimulation is seemed a little dangerous but explored anyway. The climax was disappointing. Too much activity of a slightly scary sort. Never again at this level.
EXTENSIONS AND COMMENTARY : How does one classify this kind of compound? It doesn't seem to be a psychedelic, at least at the levels reported. A stimulant? There were no mentions made of any increase in cardiovascular activity. It sounds like an example of a tactile stimulant, not for treatment of impotence but with the potential of augmenting and enhancing sexual pleasure.
From the structure activity point of view, it seems that the methyl group on the indolic 2-position again allows oral activity of something that without it, would not be. Here the parent compound is DMT, and the other examples were 2-Me-DET and 5-MeO-TMT. But of these, 2-Me-DMT seems to be the most free of "negative" side-effects, except for the sound distortion. And for the sexual stimulation, to the occasional shaker manqué amongst us who would considered that also as a negative.
| [ |
[Main Index] | [Forward |

