
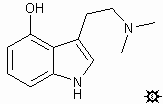
#18. 4-HO-DMT / 4-HO-DMT PHOSPHATE ESTER
4-HO-DMT; TRYPTAMINE, 4-HYDROXY-N,N-DIMETHYL; 4-INDOLOL, 3-[2-(DIMETHYLAMINO)ETHYL]; N,N-DIMETHYL-4-HYDROXYTRYPTAMINE; 3-[2-(DIMETHYLAMINO)ETHYL]-4-INDOLOL; CX-59; PSOH; PSILOCIN4-HO-DMT PHOSPHATE ESTER; TRYPTAMINE, N,N-DIMETHYL-4-PHOSPHORYLOXY; 4-INDOLOL, 3-[2-(DIMETHYLAMINO)ETHYL], PHOSPHATE ESTER; N,N-DIMETHYL-4-PHOSPHORYLOXYTRYPTAMINE; 3-[2-(DIMETHYLAMINO)ETHYL]-4-INDOLOL, PHOSPHATE ESTER; CY-39; PSOP; PSILOCIN, PHOSPHATE ESTER; PSILOCYBIN
|
| [3D .mol structure] |
A suspension of 0.38 g LAH in 10 mL anhydrous THF was held in an inert atmosphere and vigorously stirred. To this there was added, dropwise, a solution of 0.55 g of 4-acetoxyindol-3-yl-N,N-dimethylglyoxylamide in 10 mL anhydrous THF at a rate that maintained a gentle reflux. After the addition was complete, the refluxing was maintained for an additional 15 min, the reaction mixture cooled to 40 °C, and the excess hydride destroyed by the addition of water diluted with a little THF. The reaction mixture was filtered free of insoluble material under a N2 atmosphere, the resulting solids washed with THF. The filtrate and washings were combined and stripped of solvent under vacuum. The residue was distilled in a KugelRohr apparatus and the solid distillate recrystallized from EtOAc / hexane to give 3-[2-(dimethylamino)ethyl]-4-indolol (4-HO-DMT, psilocin) as white crystals which, after recrystallization from ethyl acetate / hexane, had a mp of 103-104 °C. [Erowid Note: The Erowid Expert Network has indicated that the melting point for psilocin should be closer to 170C than to Shulgin's 103-104C in TiHKAL. Merck 12th Edition lists the melting point for psilocin as 173-176C (plates from methanol). (Jan 16, 2013)] The final weight was 0.23 g (yield 56%). IR (in cm-1): 686, 725, 832, 991, 1040 and 1055; the OH stretch is at 3240. MS (in m/z): C3H8N+ 58 (100%); parent ion 204 (15%); indolemethylene+ 146 (3%); 159 (2%).
Most of the early syntheses of psilocin and psilocybin employ the O-benzyl ether as a protecting group. This provides more stability to the chemical intermediates, but also requires the additional step of reductive debenzylation. The flow chart of this process is: conversion of 4-hydroxyindole to 4-benzyloxyindole via the sodium salt, with benzyl chloride; the conversion of this with oxalyl chloride to 4-benzyloxyindole-3-glyoxylchloride; the conversion of this to 4-benzyloxy-3-(N,N-dimethyl-glyoxamide with anhydrous dimethylamine; the conversion of this to 4-benzyloxy-N,N-dimethyltryptamine with LAH in dioxane; and finally the conversion of this to 4-HO-DMT (psilocin) with hydrogen with a Pd catalyst on Al2O3. The phosphate ester, psilocybin, requires two additional steps: the conversion of 4-HO-DMT (as the sodium salt) to 4-(O,O-dibenzylphosphoryloxy)-N,N-dimethyltryptamine, with dibenzyl chlorophosphonate, followed by the catalytic removal of the benzyl groups with hydrogen and Pd on Al2O3 to give the phosphate ester of 4-HO-DMT (psilocybin). This product is much more stable in air than psilocin, and is water soluble. The yields of this conversion are, however, very bad, often less than 10%, and the two products appear to be pharmacologically equivalent. Further, I have heard that the phosphorylating agent dibenzyl chlorophosphonate must always be used in solution as it is quite unstable as a pure reagent. The fingerprint infra-red spectrum for psilocybin shows (in cm-1): 752, 789, 806, 858, 925 and the P=O stretch at 1110; the acidic OH stretches are broad peaks at 2400, 2700 and 3200. The mass spectrum is identical to that of psilocin.
DOSAGE : 10 - 20 mg, orally (as the indolol, the acetate or the phosphate)
DURATION : 3 - 6 hrs
QUALITATIVE COMMENTS : (with 6.6 mg phosphate ester, orally) "Something has started but I decide to join in a full dinner anyway. The effects develop right through the meal, with some hints of animal faces in the pork-chop bones. No movement, nothing flows, but it probably wouldn't take much effort. Another hour and I am dropping off already. The food? Somehow I doubt it. I would be completely unable to tell this from, say, 80 milligrams of MDMA except that I had a good appetite."
(with 7 mg, orally) "Basically I am not in a pleasant place -- quite neurotic -- inwardly turned -- a touch of despair -- considerable visual activity and if I were with someone I might find some sort of reinforcement. The apathy and unpleasantness is ebbing now. My mood might have been negative, and the psilocybin simply amplified everything. There was some intensification of the lights and darks around me."
(with 10 mg, orally) "Approximately forty minutes after the start, there was a flutter and a very high, stimulated feeling, and gradually things began to move very rapidly. It was astounding. When I closed my eyes I saw so many fantastically beautiful patterns, textures, colors. Everywhere I looked, eyes open, the colors were brilliant. The house looked absolutely gorgeous and nature was simply spectacular. It was a little frightening, almost too exciting, after the gentleness of other substances. I could not believe that I was doing it, and that I had the power within myself to see such beauty. I don't know how long this went on but the motion was so rapid that I felt a sort of motion sickness. Then I became quite nauseated and remained nauseated the rest of the day, until things quieted down in the evening, and then I felt absolutely wonderful."
(with 15 mg, orally) "My 'early warning system' alerted me at fifteen minutes, then all was quiet for a while. I start building up again, and I am awfully glad that I am familiar with this transition. Visual distortions. Things distract me. I can't find the cap to my pen -- must I keep writing forever? At this point I couldn't drive, let alone write, and it is just a bit more than a half hour since I took it. The furniture in my office is moving up and down. I lie down, and close my eyes. THIS is where it is at. Visuals are wild. Even with eyes open, with no visual target, there are imaginative visual effects. I imagine a dark room with a fire place going in the middle of the night, with no other inputs, and with my eyes closed I have the body image of being seated in front of that fire and I am amazed by the hallucinations and distortions I am seeing there only there is no fireplace as I am still lying in my darkened bedroom. Sort of a 2x removed hallucination. This is a night-time drug -- the day-light washes everything out. I tried but could not repeat the fireplace thing, and must be dropping rapidly. At three hours I ask if I would try some other experiment. OK, but there are some reservations. At four hours, no reservations."
(with 15 mg, orally) "As soon as I felt the chill and the alert, I lay down and closed my eyes. Indian motif. Abundant fruits, vegetables, leaves, straw, wood, vines. Very responsive sexually. Beautiful, stern, rich encounter with livingness and Indian Gods and serenity. Color and peacefulness. A couple of hours, then elaborateness dropped slightly. At this point top of temple easy, but it was a South American temple, with earth floor, straw, vines full of fruit. Familiar feeling. We are naked and we are children-adults, daring to be there, regarded benignly (stern, amused) (rising through the floor). This is one of the true ones, this plant experience.
(with 12 mg phosphate ester, intramuscularly) "This is strong. There were a lot of wild images in about two hours, and I thought that the day would never end. At about six hours I knew it would, but in fact in the evening I took 100 milligrams of seconal which allowed me to drift into a fine sleep. The next day I was fine."
(with 3 mg phosphate ester, intravenously) "The effects are immediate (in 30 seconds) and I did not have the time to build up any worry -- it was simply too fast. In about an hour I was back where I started from."
(with 12 mg phosphate ester, intravenously) "I had had eight milligrams earlier, with a very good reaction. Here, today, I feel that everything has disintegrated, and I am extremely anxious. I am very confused."
Psilocybe cubensis: (with 1.5 g, orally) "At best, some speckled patterning with my eyes closed, and in general a light intoxication. Certainly not the sparkle of LSD. Dropped quickly and felt heavy and tired, good sleep."
(with 3.5 g, orally) "Took a gram to start with, and it started in ten minutes, but not strong enough, so did the other 2.5 grams. Everything was coming at me in waves, boxing me in, the visuals were in waves and in dark earth colors, orange and brown, not the wide spectrum of acid. I was sea-sick, and vomiting helps some, and a little dope quieted the tummy. Started dropping, and everything became very good, and by midnight I was out. No hangover at all."
EXTENSIONS AND COMMENTARY : There are two generalizations implicit here, one of which I am quite at peace with, but the other is both complex and disturbing. The OK item is the casual equation between the hydroxy compound psilocin, the acetate ester, and the phosphate ester, psilocybin. As I had discussed in the CZ-74 to CEY-19 entries in 4-HO-DET, there is no proof that the ester goes to the indolol metabolically, but it is a good guess, and there have been no demonstrated differences in their pharmacology. Ditto here, with psilocin and psilocybin. I have explored both of them as pure chemicals, and I find them completely interchangeable as to their pharmacological properties.
The second generalization is more difficult and leads into some uncomfortable areas. This is the effort to equate the chemicals, psilocin and psilocybin, with their natural sources, the mushrooms. Part of the uncertainties I feel are related to the unknowns that are intrinsic to the plant sources. There are many species that have been offered and accepted as magic mushrooms. Identification in the field is one thing, but what can be said of dried, ground up plant material of unknown sources? What are they? How have they been preserved? What is their composition? The older samples may be reasonably free of the rather unstable psilocin, but psilocybin is much more stable and may persist. But so might its congeners such as baeocystin and norbaeocystin which are scattered in widely different proportions in many species, and which are quite unexplored pharmacologically. There are so many uncontrollable variables in the mushroom area that here I cast my vote for exploration with the chemicals themselves. They can, at least in principle, be analyzed, and weighed. But this is a luxury not available to many, as the syntheses of these alkaloids is difficult, and woefully illegal.
Which brings us back to the mushrooms, and the topic of the law. In the original writing of the Controlled Substances Act of 1970, our Federal drug law, there are only four plants listed as being "Scheduled Drugs." In Schedule I there was Marijuana (later defined as the plant Cannabis spp.) and Peyote (later defined as the botanical Lophophora williamsii); in Schedule II there was Opium poppy and poppy straw, and Coca leaves. It is generally known that commercial opium comes from the plant Papaver somniferum and that commercial coca comes from the plant Erythroxylon coca, but I am not aware of either of these botanical binomials having been explicitly named in the statutes. A couple of quickies were slipped in, not completely properly, in the giving of the binomial of Tabernanthe iboga as a synonym for ibogaine, and the giving of the binomial of Catha edulis as a synonym for cathinone, both Schedule I drugs. So there are definitely four, and maybe six, plants that can be considered Scheduled drugs.
But nowhere in the legal archives of current drug statutes can you find mention of Genera such as Psilocybe, Stropharia, Paneolus or Inocybe. Nor of the dozens and dozens of species that stem from them. So, you would logically conclude that these magic mushroom are not illegal? Well, yes and no.
No, in the letter-of-the-law sense that they are not explicitly named as illegal entities. But yes, in the de facto exercise of the law. With the inescapable fact that both psilocin and psilocybin are named as Schedule I drugs, and the acknowledgment that there are some mushrooms that might contain these drugs, then these botanical entities become legal complications. Might the dried fruiting bodies be seen as packaging strategy for the sale and delivery of a Scheduled I drug? Might the growing of them be seen as a production strategy for the manufacture of a Schedule I drug? Of course it might be, as the law has stated that the manufacture and sale of Schedule I drugs is a Federal felony. "Your Honor. I gathered these things out there in the field for my dinner salad. I had no idea that they contained something illegal." A reasonable defense, and it may well work today, along with the argument that opium poppy pods are buyable at the Farmer's Market as floral decorations, and morning glory seeds can be bought at the local nursery for next Spring's garden. Innocence may be a virtue for a while, as it is not widely recognized that these decorative poppies are in fact Schedule II opium capsules and those Ipomoea seeds in fact contain ergine, a Schedule III depressant. But that is today. What happens tomorrow?
Today, to a large measure, the burden of proof still falls upon the accuser, and that ephemeral and undocumented "presumption of innocence" concept provides some measure of protection. They, the accusors, must prove you are guilty. But, as the legal structure drifts from the criminal statutes to the regulatory statutes, this protection is lost. You must prove that you are innocent. The perfect example is the random urine test, which demands, without any probable cause, that you prove that you do not have drugs in you. There is no presumption of innocence. This has been the sad state of our income tax laws for years, and now it is becoming a reality in our drug laws. Prove to the court that you didn't know that these mushrooms were psychoactive! Shades of the Inquisitions of a few hundred years ago. Or the Salem travesties of more recent times. Prove to us you are not a witch.
There is quite a body of scientific literature that discusses the changes (increases and well as decreases) of psilocybin and psilocin content in mushrooms as a function of their nutrient diet. And, under the 4-HO-DET entry, I mentioned that the inclusion of an unnatural component into the diet just might produce an unnatural alkaloidal product, with an exploitation of the natural and available enzyme systems that are part of the mycelial structure.
Another aside. There is a trivial, and fun, bit of nomenclature which I have used for years. I have, in my notes, referred to psilocybin as PSOP (because of the phosphate thing) and psilocin as PSOH (because of the exposed OH group). I have gotten into the habit of referring to the acetate as PSOA, the O-methyl ether as PSOM and the chemical intermediate O-benzyl ether as PSOB. I know that this will never catch on, but I still do it because it is convenient and a bit campy. One code that is not mine, but Sandoz's, is CMY for 1-methyl-psilocin. I know it has been looked at in a clinical environment, but I have not idea as to its activity. It is a simple thing to make. I would love to know what it does.
| [ |
[Main Index] | [Forward |

