
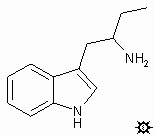
#11. a-ET
ALPHA-ETHYLTRYPTAMINE; INDOLE, 3-(2-AMINOBUTYL); TRYPTAMINE, ALPHA-ETHYL; 3-(2-AMINOBUTYL)INDOLE; MONASE
|
| [3D .mol structure] |
A suspension of 31.7 g LAH in 300 mL anhydrous THF, stirring under an inert atmosphere, was treated by the addition of a solution of 36 g 1-(3-indolyl)-2-nitrobut-1-ene in 285 mL anhydrous THF. This was added dropwise over the course of 3 h, while the mixture was being brought up to reflux temperature. The reaction mixture was held at reflux for an additional 2 h, then allowed to return to room temperature. After standing overnight, the excess hydride was destroyed by the cautious addition of 500 mL wet Et2O, followed with 70 mL H2O, 100 mL THF, and finally 20 mL 50% NaOH. After 1 h additional stirring, the solids were removed by filtration, washed with 1.5 L Et2O, the combined filtrate and washings dried over anhydrous K2CO3, and the solvent removed under vacuum. The residue, 78 g, was dissolved in 100 mL MeOH, treated with 12 mL acetic acid, stripped of volatiles under vacuum, redissolved in a mixture of 250 mL ethyl acetate and 30 mL MeOH, concentrated to a volume of about 100 mL, and again treated with 2 mL acetic acid. The product a-ethyltryptamine acetate (a-ET) separated as a solid with a mp of 164-165.5 °C. Anal. (C14H20N2O2); H: calcd, 8.11; found 7.60: C,N. It can be recrystallized from ethyl acetate/MeOH which increased and tightened the mp to 165-166 °C. The free base, from ethyl acetate/petroleum ether, had a mp 97-99 °C. The hydrochloride salt has a mp 215.5-218 °C. The picrate had a mp 165-166 °C.
DOSAGE : 100 - 150 mg, orally
DURATION : 6 - 8 hrs
QUALITATIVE COMMENTS : (with 50 mg, orally) "No effects of any kind were felt with 50 milligrams, orally."
(with 100 mg, orally) "A nearly imperceptible feeling of well-being and pleasure was noted about 80 minutes later, and seemed completely gone at three hours.'
(with 105 mg, orally) "Very slowly soluble in water and mildly bitter. I was aware of something just before a half-hour, and at one hour I had a light-headed sparkle and felt light of body. It is like speed without the cardiovascular, or like a psychedelic without the visuals. I can see how it was sold in Chicago as MDMA. At two hours, a slight cooling of the feet, a bit of unsureness in the gut, a tendency to squeeze the teeth together, a trace of eye-wiggle, and a tendency to talk with my ears popped. Four and a half hours, largely out, with some residue in eyes and teeth. Six hours baseline, and fine sleep. No residue."
(with 110 mg, orally) "I am in a very different place. It's exciting but at the same time I don't know what to do with the energy. It makes my eyes want to close."
(with 120 mg, orally) "Very keen, pure euphoria, feels great. Reaches +3 in about one hour. Sharply focused feelings very strong, strong energy push. Keeps rising until it goes over the top and begins to break up. The pure tone of euphoria gets joggled with other feelings, like a bit too much to handle. Not really uncomfortable, but not as nice as the earlier +2 stage. A wall seems to grow around me. I am being shut off from intimate contact with others. But in a couple of hours the push of the drug diminishes, and I get more comfortable. The day ended beautifully."
(with 130 mg, orally) "There was a smooth onset of relaxation at about 55 minutes with only a trace of motor intoxication. Both radio and television seemed more enjoyable than normal and there was a definite enhancement of the beauty of instrumental music. The effect seemed to peak at about 150 minutes and was essentially gone at the five hour point. There were never any visuals nor any type of sensory distortions, just warm pleasant feelings. No interference with sleep was noted, and there were no after-effects the next day."
(with 150 mg, orally) "My dosage level was the highest of the group, but to my surprise, it had almost no effect whatsoever. A plus-one, if anything. After the peak, as I was slowly coming down, I was aware of feeling slightly depressed. This state continued until I achieved baseline, but was not severe enough to prevent me from participating in the general good spirits of the group. There is a real possibility that my weekly use of MDMA for writing might have built up a tolerance to the stimulation of this material. I think that that may be close to the answer. Would I take it again? Not with much enthusiasm. It didn't give enough exciting rewards."
(with 160 mg, orally) "A strong feeling of being-at-peace was evident in an hour, although there was some concentration required to do things in a coordinated way. I wouldn't want to drive a car. There seemed to be very easy drifting of thoughts but no visuals or sensory distortions. There were no GI disturbances anywhere along the line except for some loose stools the next morning. Appetite was slightly depressed, but food tasted very good. Sex at the 2-hour point showed some difficulty in reaching orgasm but significantly enhanced pleasure during orgasm once it was attained. A very slight tremor could be detected in the fingers around the peak of the experience. There was a desire to talk with friends somewhat reminiscent of MDMA; I am sure that this drug could be quite a social-enhancing material. The effects wore off gradually and were essentially gone by the six hour point. Sleep was unaffected, however the next morning there was a slight feeling of dullness and possibly hang-over which quickly wore off."
EXTENSIONS AND COMMENTARY : This base, a-ET or etryptamine, was a promising anti-depressant, explored clinically as the acetate salt by Upjohn under the name of Monase. Its central stimulant activity is probably not due to its monoamineoxidase inhibition activity, but appears to stem from its structural relationship to the indolic psychedelics. It was withdrawn from potential commercial use with the appearance of an unacceptable incidence of a medical condition known as agranulocytosis, but the extra mural research into its action, among the lay population, goes on.
One property has been mentioned more than once in anecdotal reports. It appears to serve well, with short term dosage regimens, as an effective tool in kicking dependency on opiates. In chronic use, there is a rather rapid tolerance built up over four or five days, that allows a dosage escalation to a daily load of a gram or more. There might be some discomfort such as sores in the softer tissues of the mouth, but apparently the withdrawal from heroin is easy and effective. Here is a potential tool in addiction treatment that might warrant closer investigation.
Other homologues of a-ET have been synthesized. The a-propylhomologue (a-PT) has been made from tryptophan, and the acetate salt was recrystallized from ethyl acetate/MeOH and melted at 158-158.5 °C. It has not, to my knowledge, ever been tasted. But I suspect that it will take a pretty hefty dosage to get some CNS effect based on the loss of potency with the similar homologation in the Muni Metro series related to MDMA. Rather than lengthening the chain on the alpha-position, some studies have exploited the known potency enhancement that comes from putting a methoxyl group on the 5-position of the indole. This compound, 5-MeO-a-ET, has been made from the 5-methoxyindole-3-aldehyde by coupling with nitropropane (with ammonium acetate) to form the nitrobutene which is a reddish crystalline material, mp 114-116 °C from ethanol. LAH reduction in Et2O/THF gave the desired 5-MeO-a-ET in a 72% yield, mp 201-203 °C as the hydrochloride salt. An alternate synthesis that avoids LAH involves the conversion of 5-methoxyindole to the nitrobutane with 2-nitro-1-butene, followed by reduction with nickel boride to give 5-MeO-a-ET, as the free base in a 52% yield, mp 110-112 °C. As might have been predicted, it was more potent than a-ET by a factor of two with 70 milligrams orally producing a trippy feeling that lasted several hours accompanied with an increased heart beat and difficulty in sleeping. There were no psychedelic effects as such, and no unpleasant side effects. Another compound that has been closely associated with a-ET is a carboline. If a molecule of acetone is brought to react with the amine group and the indolic 2-position, in a condensation that is called a Pictet-Spengler reaction, there would be formed 1,1-dimethyl-3-ethyl-1,2,3,4-tetrahydro-b-carboline. This is a chemical ally of the harmine family of alkaloids, but I have not heard of its having been explored psychedelically. It has been reported to be an impurity of commercial a-ET (including the prescheduling product from the Aldrich Chemical Company) to an extent of some 30%. At these levels, it was suggested that it might play some role in the central action of the parent tryptamine.
a-ET has played yet another role in the evolution of our drug laws, a role that will be found to be of extraordinary importance once it becomes more widely known. This compound may prove pivotal in our ultimate definition of the Analogue Drug Law. I want to talk about: (1) The Controlled Substance Analogue Drug Bill; (2) What happened in a trial in Denver; and (3) What happened in a District Court in Colorado.
During the most political period of the War on Drugs, Congress passed, and the president signed, a new law every two years, on the even-numbered years (the years of congressional re-election) that increased either the definition of what were illegal drugs, or the penalties that follow a conviction for having been associated with them in any way. In 1986, there was a proposed draft of a bill called the "Designer Drug Bill" that had been created within the DEA, and sent on to the Justice Department who, in turn, submitted it to Congress as desired legislation. This was a proposal that would make illegal the tinkering with the structure of a molecule of an illegal drug, to change it in a way that would make it fall outside of the explicit listings of illegal drugs but without significant changes in its pharmacological effects. It was the first time a drug law would define a crime by the activity of a compound as well as by chemical structure. The proposal went to the appropriate legislative committee and, with some modifications, it became law in 1986. There was considerable celebration within the DEA, expressing a "We did it!" kind of satisfaction.
The first three Articles of the Constitution of the United States are entitled: Article. I. The Legislative Department; Article. II. The Executive Department; and Article. III. The Judicial Department. The first of these, consisting of Congress, has the role of writing law and defining the military structure of the nation. The second of these defines the president, who approves the laws of Congress and is the highest military officer. The third of these is invested in the enforcement of these laws. The three departments were defined in a way to assure a balance of power. It is a dangerous step towards a totalitarian state when one special interest group (here the DEA) can, in effect, both write the law and then enforce it.
Here is the text of the Analogue Drug Bill:
(1) The Controlled Substance Analogue Drug Bill. This is contained within Public Law 99-570, the Controlled Substances Analogue Enforcement Act of 1986. This is the so-called "Designer Drug" bill which was intended to allow the prosecution of any act associated with an unscheduled drug, if that drug is analogous either in structure or in action to a scheduled drug, and if it is intended for use in man. Here is the exact wording of this amendment:
This is the exact wording of the law, and I have discovered that the more times I read it the more convinced I become that, whatever the original intent might have been, it was structured in a way to promote vagueness. I have written elsewhere about the rhetorical nightmare of a double disclaimer, "substantially similar." "Similar" means "pretty much the same." "Substantially identical" would means "pretty much the same." But what does "substantially similar" mean? I like the analogy of seeing two cut glass shakers in the center of the fancy table, one with small holes in the silver screw-down cap containing salt, and the other with slightly larger holes containing pepper. Are these two items substantially similar? If you happen to be a collector of antique crystal glassware, these items are completely identical. If you happen to need to add a condiment to your entree these items are totally different. You must know whose eyes are being looked through to approach the question of "substantial similarity." At a trial a few years ago in Southern California the issue was settled once and for all for a confused jury when a forensic chemist gave an expert opinion that two things were substantially similar when they were greater than 50% identical. Is the right hand more than 50% identical to the right foot? This opinion was patently absurd.(32)(A) Except as provided in subparagraph (B), the term 'controlled substance analogue' means a substance --
(i) the chemical structure of which is substantially similar to the chemical structure of a controlled substance in Schedule I or II;
(ii) which has a stimulant, depressant, or hallucinogenic effect on the central nervous system that is substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in Schedule I or II; or
(iii) with respect to a particular person, which such person represents or intends to have a stimulant, depressant, or hallucino-genic effect on the central nervous system that is substantially similar to or greater than the stimulant, depressant, or hallucinogen effect on the central nervous system of a controlled substance in schedule I or II.
(B) Such term does not include --
(i) a controlled substance;
(ii) any substance for which there is an approved new drug application;
(iii) with respect to a particular person any substance, if an exemption is in effect for investigational use, for that person, under section 505 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 355) to the extent conduct with respect to such substance is pursuant to such exemption; or
(iv) any substance to the extent not intended for human consumption before such an exemption takes effect with respect to that substance.
SEC. 203. A controlled substance analogue shall, to the extent intended for human consumption, be treated, for purposes of this title and title III as a controlled substance in Schedule I.
(2) What happened in a trial in Denver? A few years ago a young man discovered that the Aldrich Chemical Company offered alpha-ethyltryptamine acetate as a fine chemical. He could buy it in 100g quantities, and package it in 150 milligram capsules to be sold to the street trade as Ecstasy, or MDMA. He could and he did. His actions came to the attention of Law Enforcement, and an opinion was obtained from a DEA chemist that a-ET was not an analogue substance. So the prosecutor decided against pressing charges. But not every one agreed with this not-analogue opinion.
So the chemist solicited the thoughts of his professional colleagues and the answers cam back with as many no's as yes's. The no's were from those who reasoned objectively (scientific, compare the structures) and the yes's were from those who reasoned subjectively (abuse potential, compare the action).
The adventurous a-ET peddler continued, and was again brought to task. The analytical duties went to another chemist, and charges were finally brought under the Analogue Drug Bill. But the earlier opinion was in the record, and the first chemist was brought in by the defense to present these findings at the trial. Clearly there was uncertainty if this was an analogue of anything that was scheduled. The research toxicologist for the home-office of the DEA gave testimony that it was, without question, an analogue. But on cross examination, he was asked just how many times, and for how many different drugs, he had been asked that same question, as an expert witness at a criminal trial. Perhaps twelve, he said. And how many times had he offered the conclusion that the proposed compound had been an analogue of a scheduled drug? In every case. The judge decided that there were some conflicting opinions here, amongst the experts, and dismissed the charges. The defendant was given the warning that this kind of leniency was not common and told to behave himself in the future.
(3) The text of the appellate decision in this matter is a valuable lesson in the fine aspects of grammatical analysis. This is all from 806 F.Supp. 232 (D.Colo., 1992). In way of background it emphasizes that the purpose of the controlled substance analogue statute is to attack underground chemists who tinker with molecules of controlled substances to create new drugs that are not yet illegal. In this case, the defendants were not chemists who created or marketed a designer drug but rather allegedly purchased and distributed a substance that preexisted drugs to which it was a purported analogue. This was probably, in and of itself, sufficient reason to deny the appeal. But the argument developed marvelous new texture as things progressed. As a reminder of the wording of the law (here SS is, of course, substantially similar but this terminology is not addressed in the decision), the three phases of the definitional part of the law can be summarized as follows:
(i) a chemical structure which is SS to ... ;
(ii) which has an effect that is SS to ... ;
(iii) which is represented as having an effect that is SS to ...
The prosecution's reading and analysis of this definition:
"The government's reading of the analogue definition has superficial appeal. As a matter of simple grammar, when an "or" is placed before the last term in a series, each term in the series is usually intended to be disjunctive. Under this reading, a-ET would be an analogue if it satisfies any of the three clauses; however, this reading ignores other grammatical principles that apply in favor of defendant's construction. The operative segments of clauses Iii) and (iii) both begin with the word 'which,' signaling the start of a dependent relative clause modifying a previous noun. In each case the precedent noun is 'chemical structure' found in clause (i). Because both clauses (ii) and (iii) can be read to modify clause (i) the statutory language can be fairly read as requiring the two-pronged definition asserted by the defendants."
The defendant's reading and analysis of this definition:
"Defendant's reading is also bolstered by a deeply rooted rule of statutory construction. A statute must be construed to avoid unintended or absurd results. If I adopt the government's construction and read clause (ii) independently, alcohol or caffeine would be controlled substance analogues because, in a concentrated form, they can have depressent or stimulative effects substantially similar to a controlled substance. Likewise if I read clause (iii) independently, powdered sugar would be an analogue if a defendant represented that it was cocaine, effectively converting this law into a counterfeit drug statute. In both cases the defendant could be prosecuted for selling a controlled substance analogue even though the alleged analogue did not have a chemical structure substantially similar to a schedule I or II controlled substance. Therefore, to prevent this unintended result, clause (i) must apply to any substance that the government contends is a controlled substance analogue."
There is a most instructive bit of history to be considered. In July, 1986, the House of Representatives considered the Designer Drug Enforcement Act of 1986 (H.R. 5246). As with the Senate, the House bill focused on underground chemists who seek to evade the drug laws by slightly altering a controlled substance. The House proposed a two-pronged definition of "analogue" that is virtually identical to the construction advocated by the defendant here. The House bill contained the same three clauses as the current statute, but added the word "and" after clause (i). Congress ultimately adopted the analogue statute as part of the comprehensive "Anti-Drug Abuse Act of 1986." Inexplicably, the analogue definition enacted by Congress dropped the word "and" after clause (i).
This pretty well defines the legislative intent of Congress, and I would give a pretty penny to meet the writer who happened to delete that "and," the one critical word that changed the heart of the law. i would like to know to whom he answered.
Here is a masterpiece of logic which makes some sense out of sloppy law. It must be remembered that the purpose of all of this is to determine if one, or two, or three definitions must be applied to establish just what is an analogue. This court declared that a substance may be a controlled substance analogue only if it satisfies clause (i) and at least one of clauses (ii) or (iii).
There is a fascinating, and potentially most disruptive, appeals ruling made in 1996 concerning the interpretation of this law, in this case involving aminorex and phenethylamine as being analogues of 4-methyl aminorex and methamphetamine, respectively, and thus chargeable as a crime under this analogue statute. This is from the United States District Court for the District of Minnesota, No. 95-2132. In this ruling the Analogue Drug Bill is paraphrased with the following text: "... a drug becomes a controlled substance if it has a chemical structure substantially similar to that of a controlled substance, and either has a substantially similar effect on the user's central nervous system, or a relevant someone represents that it has or intends it to have such an effect." This is fascinating in that the source cited for this quote, 21 U.S.C. SS 802(32)(A), has no such text. And it is potentially disruptive for two reasons. It suggests that an analogue shall become a controlled substance, rather than be treated as if it were a controlled substance. It also introduces a new and undefined term, a "relevant someone." I do not have the legal background to guess the extent that this statement can influence future court challenges in the area of controlled substances analogues. Do, always, keep in mind that the finding that a chemical, in a given situation, is a controlled substance analogue does not make that chemical a controlled substance. The analogue status exists for just the single instance, and the next time the arguments all start over again.
Back to the case involving a-ET. The DEA retreated, licking its wounds, and got its own back by immediately proposing the placement of a-ET into Schedule 1. They succeeded, and Monase is today no longer an FDA-approved antidepressant but it is, instead, a drug with a high potential for abuse. One of the more unexpected forms of abuse can be seen in the costs to the researcher who wished to study it in some legal way. Before it became a scheduled drug, alphaethyltryptamine was what is known as a "fine chemical" and was listed in the catalog of a major chemical company (1993) for a modest $60.90 for a hundred grams. It became a Schedule I drug by emergency scheduling that same year. Recently (1995) I noted that the chemical has been discontinued (as a fine chemical) but has appeared in a catalog from a major supply house for neurological chemicals. Alphaethyl tryptamine now requires a DEA license for purchase, and retailed at $424.00 for 100 milligrams. That calculates out at $424,000.00 for a hundred grams, a price inflation of a factor of almost 7000, or a 700,000% increase. Now THAT is truly drug abuse.
| [ |
[Main Index] | [Forward |

