
#124 META-DOB
5-BROMO-2,4-DIMETHOXYAMPHETAMINE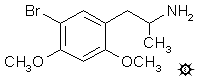
|
| [3D .mol structure] |
DOSAGE: 50 - 100 mg.
DURATION: 5 - 6 h.
EXTENSIONS AND COMMENTARY: There is very little synthetic information available, and some of it is contradictory. The initial human report in the medical literature says only that a dosage of about 100 milligrams produced effects that were similar to those produced by MDA. Both the quality of the experience and the potency of the compound have been modified in more recent publications by the originators of this compound. A 40 milligram dose, after an induction period of an hour, produced a vague uneasiness that was interpreted originally as a threshold psychedelic effect. At doses in the 60 to 90 milligram range, there were produced feelings of anxiety and paranoid fantasies, and distinct toxic signs such as flushing, palpitations, and occasional nausea, vomiting and diarrhea. Any psychedelic effects seem to have been blurred by the more obvious toxic actions of the drug. I have been told that their final conclusion was that the drug appears toxic in the 50 to 60 milligram range. I have not personally explored this positional isomer of DOB.
The positional isomer of DOB with the bromine in the ortho-position is 4,5-dimethoxy-2-bromoamphetamine and is called, not surprisingly, ORTHO-DOB. It has been made by the condensation of 2-bromo-4,5-dimethoxybenzaldehyde with nitroethane to give 1-(2-bromo-4,5-dimethoxyphenyl)-2-nitropropene with a mp of 105-106 °C. Reduction to the amphetamine had to be conducted at a low temperature and using only an equimolar amount of lithium aluminum hydride, to minimize reductive removal of the bromo group. The hydrochloride salt of 2-bromo-4,5-dimethoxyamphetamine (ORTHO-DOB) had a mp of 214-215.5 °C, and the hydrobromide salt a melting point of 196-197 °C or of 210 °C. Both have been reported. The yield from the direct bromination of 3,4-DMA was apparently very bad. I do not think that the compound has ever gone into man.
There are three other dimethoxyamphetamine isomers known, and each has been explored chemically as to its reactivity with elemental bromine. With 2,3-DMA, a mixture of the 5-Br-2,3-DMA and 6-Br-2,3-DMA was formed; with 2,6-DMA, 3-Br-2,6-DMA was formed; and with 3,5-DMA, a mixture of 2-Br-3,5-DMA and the 2,6-dibromo product was produced. The bromination of 2,5-DMA is, of course, the preferred procedure for the synthesis of 4-Br-2,5- DMA, or DOB, q.v. None of these positional isomers has evear been put into man, but 3-Br-2,6-DMA and the iodo-counterpart have been explored as potential radio-fluorine carriers into the brain. This is all discussed in the 3,4-DMA recipe.
| [ |
[Main Index] | [Forward |

