
#119 ME
METAESCALINE; 3,4-DIMETHOXY-5-ETHOXYPHENETHYLAMINE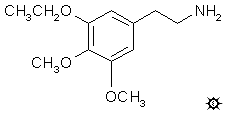
|
| [3D .mol structure] |
A mixture of 15.6 g 3-bromo-4-methoxy-5-ethoxybenzaldehyde and 10 mL cyclohexylamine was heated with an open flame until it appeared free of H2O. The residue was put under a vacuum (0.5 mm/Hg) and distilled at 148-155 °C yielding 19.2 g 3-bromo-N-cyclohexyl-4-methoxy-5-ethoxybenzylidenimine as an off-white crystalline solid with a melting point 66-68.5 °C. Recrystallization from 100 mL boiling MeOH gave a mp of 67-68.5 °C. The C=N stretch in the infra-red was at 1640 cm-1. Anal. (C16H22BrNO2) C,H.
A solution of 17 g 3-bromo-N-cyclohexyl-4-methoxy-5-ethoxybenzyl-idenimine in 200 mL anhydrous Et2O was placed in an atmosphere of He, stirred magnetically, and cooled with an external dry-ice acetone bath. Then 38 mL of a 1.55 M solution of butyllithium in hexane was added over 2 min, producing a clear yellow solution. There was then added 25 mL of butyl borate at one time, and the stirred solution allowed to return to room temperature. This was followed with 100 mL of saturated aqueous ammonium sulfate. The Et2O layer was separated, washed with additional saturated ammonium sulfate solution, and evaporated under vacuum The residue was dissolved in 200 mL of 50% MeOH and treated with 12 mL of 30% hydrogen peroxide. This reaction was mildly exothermic, and was allowed to stir for 15 min, then added to an aqueous solution of 50 g ammonium sulfate. This was extracted with 2x100 mL CH2Cl2, the pooled extracts washed once with H2O, and the solvent removed under vacuum. The residue was suspended in dilute HCl, and heated on the steam bath for 0.5 h. Stirring was continued until the reaction was again at room temperature and then it was extracted with 2x100 mL CH2Cl2. These extracts were pooled and in turn extracted with 2x100 mL dilute NaOH. The aqueous extracts were reacidified with HCl, and reextracted with 2x100 mL CH2Cl2. After pooling, the solvent was removed under vacuum to yield an oily residue. This was distilled at 118-130 °C at 0.2 mm/Hg to yield 7.5 g of 3-ethoxy-5-hydroxy-4-methoxybenzaldehyde as a distillate that set to white crystals. Recrystallization from cyclohexane gives a product with a mp of 77-78 °C. Anal. (C10H12O4) C,H.
A solution of 7.3 g of 3-ethoxy-5-hydroxy-4-methoxybenzaldehyde in 100 mL acetone was treated with 5 mL methyl iodide and 8.0 g finely powdered anhydrous K2CO3, and held at reflux on a steam bath for 6 h. The solvent was removed under vacuum, and the residue was suspended in H2O. After making this strongly basic, it was extracted with 3x50 mL CH2Cl2, the extracts were pooled, and the solvent removed under vacuum. The residual amber oil was distilled at 110-120 °C at 0.4 mm/Hg to yield 7.3 g of a white oil. This spontaneously set to white crystals of 3,4-dimethoxy-5-ethoxybenzaldehyde which had a mp of 49-49.5 °C. Anal. (C11H14O4) C,H. This same aldehyde can be obtained, but in a less satisfactory yield, by the ethylation of 3,4-dimethoxy-5-hydroxybenzaldehyde described under the preparation of metaproscaline (MP).
A solution of 7.2 g 3,4-dimethoxy-5-ethoxybenzaldehyde in 100 mL nitromethane containing 0.1 g anhydrous ammonium acetate was held at reflux for 50 min. The excess nitromethane was removed under vacuum producing 6.8 g of a red oil which was decanted from some insoluble material. Addition of 10 mL hot MeOH to the decantings, gave a homogeneous solution that spontaneously crystallized on cooling. The yellow crystals were removed by filtration, washed sparingly with MeOH and air dried yielding 3.5 g yellow crystals of 3,4-dimethoxy-5-ethoxy-beta-nitrostyrene, with a mp of 89.5-90 °C after recrystallization from MeOH. Anal. (C12H15NO5) C,H.
A solution of 2.0 g LAH in 100 mL anhydrous THF under He was cooled to 0 °C and vigorously stirred. There was added, dropwise, 1.3 mL of 100% H2SO4, followed by the dropwise addition of a solution of 3.1 g 3,4-dimethoxy-5-ethoxy-beta-nitrostyrene in 50 mL anhydrous THF, over the course of 10 min. The mixture was stirred at 0 °C for a while, and then brought to a reflux on the steam bath for 30 min. After cooling again, the excess hydride was destroyed with IPA in THF, followed by the addition of 20 mL 10% NaOH which was sufficient to convert the solids to a white and granular form. These were removed by filtration, the filter cake washed with IPA, the mother liquor and filtrates combined, and the solvents removed under vacuum. The residue was added to 150 mL dilute H2SO4, and the cloudy suspension washed with 2x75 mL CH2Cl2 which removed much of the color. The aqueous phase was made basic with 25% NaOH, and extracted with 3x50 mL CH2Cl2. The solvent was removed from these pooled extracts and the residue distilled at 103-116 °C at 0.25 mm/Hg to provide 2.3 g of a colorless viscous liquid. This was dissolved in 10 mL IPA, neutralized with about 25 drops of concentrated HCl, which produced an insoluble white solid. This was diluted with 40 mL anhydrous Et2O added slowly with continuous stirring. The white crystalline 3,4-dimethoxy-5-ethoxyphenethylamine hydrochloride (ME) was isolated by filtration, washed with Et2O, and air dried, and weighed 2.4 g. It had a mp of 202-203 °C which increased by one degree upon recrystallization from boiling IPA. Anal. (C12H20ClNO3) C,H.
DOSAGE: 200 - 350 mg.
DURATION: 8 - 12 h.
QUALITATIVE COMMENTS: (with 200 mg) It tasted pretty strong. However, the taste was soon gone, and an energetic feeling began to take over me. It continued to grow. The feeling was one of great camaraderie, and it was very easy to talk to people. Everyone was talking to everyone else. I found it most pleasant, energetic and at the same time relaxing, with my defenses down. This material did not seem to lead to introspection; however, it might if one took it without other people around. Heightened visual awareness was mild, but the audio awareness was quite heightened. The feeling of being with everyone was intense.
(with 250 mg) Initially I took 200 milligrams of metaescaline, and the experience developed for me very gradually at first, and very pleasantly. After about one half hour I became aware of a wall that seemed to shut me in, not unpleasantly. The wall slowly dissolved, but I was afraid I might get into a negative experience. I felt immediate relief (from this isolation) upon taking the additional 50 mg (at 2:23 into the experiment) as though glad of the decision. I lay down outside on a blanket. There was a marvelous feeling inside, although no imagery. I felt the wall dissolve completely, and I desired to join the group. From this point on the experience was most enjoyable, euphoric. Although not dramatic like some psychedelics, it was most rewarding for me personally. I felt a marvelous bond with everyone present, with clear-headed, excellent thinking, and excellent communication. All in all, a most rewarding and enjoyable experience. Afterwards I felt much strengthened, with good energy and good insight. I have a strong feeling that the group tailored the nature of the experience, and that I and others were most desirous of group interaction. I feel that one could do a lot of other things with it if one turned one's attention to it.
(with 275 mg) Onset of both physical and mental change was slow relative to other psychochemicals. Very gradual internal stirrings were felt at about the hour-and-a-half point. These were mostly feelingful rather than cognitive, and were quite pleasurable. At about the two-and-a-half hour point I grew quite thirsty, and drank a pint of beer. Almost immediately, and quite unexpectedly, I tomsoed to a much higher level and remained there for another three hours until the whole experience waned. [The verb, to tomso, means a sudden rekindling of the drug-induced altered state with a small amount of alcohol. It is explained in the recipe for TOMSO.] During the experience heights, and in fact before it reached its height, talking was easy and unimpeded. The transference feelings so characteristic of MDMA were basically not there. But for purposes of psychotherapy, there were some advantages: fluent associations, undefended positions, and general bonaise.
(with 400 mg) Ingested 300 milligrams at about 1:30 in the afternoon. Very quiet climb. Occasional yawns. Matter-of-fact view of the world. No rosy glow. At the end of the second hour, I seem to be stuck at a ++. Take another 100 milligrams at 3:45 PM. Still tastes awful. Feel a small head-rush fifteen minutes after taking the supplement, and within a half hour I am completely +3. For a while this was a sterner mescaline. Saw the eternal, continual making of choices, all opposites continually in motion with each other. Yin and yang everywhere, giving life to every molecule. The universe itself keeps alive by the action-reaction, the yes-no, the black-white, male-female, plus-minus. All life is a continual making of choices on all levels. Then I closed my eyes, and I found myself floating up to the very top of a temple, where there was radiant light and a sense of homecoming. Making love is a clear stream over and through rocks and canyons--the earth and sky make love, and the rocks make love to other rocks, and the water is the teasing, fondling, living and moving actions of loving. To realize that, on some level, all existence makes love to all other existence. The Japanese Garden: a structured way of laying out a small glimpse into cosmic love-making, so that it can be read by other human souls. All loving, when direct and free and undemanding, is a touching of the Source. The hardest lesson, of course, is how to love yourself that same way. And it remains both the first lesson of Kindergarten and the Ph.D. final. I was able to drift into sleep at about 4:00 AM.
EXTENSIONS AND COMMENTARY: The reorientation of the single ethyl group of escaline (E) to the meta-position produces metaescaline (ME). In cats, in studies of over 50 years ago, the two compounds produced similar effects at similar dosages. In man, ME also appears to be similar to mescaline in potency. However, a subtle difference is apparent between ME and Peyote, the natural source of mescaline. With Peyote itself, the initial taste of the crude cactus is more than just foul; it might better be described as unbelievably foul. But in the middle of a Peyote experience, the taste of the cactus is truly friendly. When ME was retasted in the middle of an experience, the taste was still foul.
There are other distinctions from mescaline. Unlike mescaline or Peyote, there is rarely any body discomfort during the early phase of intoxication, no nausea and only an occasional comment suggesting hyperreflexia. And, also unlike mescaline, most subjective reports on ME claim that music produces little imagery, and the exaggeration of color perception is more reserved. Appetite is normal, the tastes and textures of food are unusually rewarding. No subject has ever expressed a reluctance to repeat the experience. Sleep is easy, refreshing, and the following day seems free from residue.
| [ |
[Main Index] | [Forward |

