
#113 MDMP
a,a,N-TRIMETHYL-3,4-METHYLENEDIOXY-PHENETHYLAMINE; METHYLENEDIOXYMEPHENTERMINE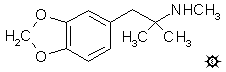
|
| [3D .mol structure] |
DOSAGE: above 110 mg.
DURATION: perhaps 6 hours.
QUALITATIVE COMMENTS: (with 60 mg) There was a faint, dull alerting at just over a half hour. The time sense was out of order, and an absence of visuals but a generalized attentiveness to my surroundings was suggestive of MDMA. Nothing remained at the six hour point.
(with 110 mg) There was a light-headedness, and a complete absence of libido. Nothing in any way psychedelic, but there are hints of discomfort (jaw tension) that will bear close watching at higher dosages. It might evolve at higher levels into something like MDMA.
EXTENSIONS AND COMMENTARY: This is one of several candidates for clinical use as a substitute for MDMA, but there will have to be a much broader study of its qualitative action in man. It is clearly not psychedelic at these modest levels, and in in vitro animal studies it was apparently inactive as a serotonin releaser. The warped logic for looking at phentermine analogs was discussed in the comments that concerned MDPH. The initials used here have been chosen with care. MDM should not be used as it has found some currency as an abbreviation for MDMA (Methylene-Dioxy-Methamphetamine). MDMP fits neatly with Methylene-Dioxy-Me-Phentermine.
| [ |
[Main Index] | [Forward |

