
#41 2C-T-4
2,5-DIMETHOXY-4-(i)-PROPYLTHIOPHENETHYLAMINE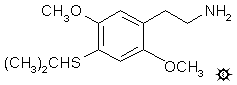
|
| [3D .mol structure] |
A mixture of 4.8 g POCl3 and 4.5 g N-methylformanilide was stirred and allowed to stand at room temperature for 1 h To this claret-colored solution was added 3.0 g of 2,5-dimethoxyphenyl isopropyl sulfide, producing an exothermic reaction and immediate reddening. This was heated for 0.5 h on the steam bath, then quenched in 200 mL of warm H2O producing immediate crystals. Stirring was continued for a few min, and then the solids were removed by filtration, washed with H2O and sucked as dry as possible. When they were ground up under an equal weight of cold MeOH, refiltered and air dried, they gave 2.35 g of 2,5-dimethoxy-4-(i-propylthio)benzaldehyde as pale yellow solids (in some runs this was a pale lime-green color) with a mp of 89-90 °C. A wasteful recrystallization from MeOH gave pale yellow crystals with a mp of 90 °C sharp.
To a solution of 6.7 g 2,5-dimethoxy-(i-propylthio)benzaldehyde in 40 g of nitromethane there was added 0.10 g of anhydrous ammonium acetate, and the mixture was heated on the steam bath for 2 h. The excess reagent/solvent was removed under vacuum yielding 8.9 g of orange solids. This was recrystallized from 200 mL boiling MeOH providing 6.2 g of 2,5-dimethoxy-beta-nitro-4-(i-propyl-thio)styrene as lustrous golden orange platelets.
A solution of LAH (80 mL of a 1 M solution in THF) was cooled, under He, to 0 °C with an external ice bath. With good stirring there was added 2.1 mL 100% H2SO4 dropwise, to minimize charring. This was followed by the addition of 5.74 g 2,5-dimethoxy-beta-nitro-4-(i-propylthio)styrene as a solid, a bit at a time. After 15 min further stirring, the temperature was brought up to a gentle reflux on the steam bath for another 15 min, then allowed to stand at room temperature overnight. After cooling again to 0 °C, the excess hydride was destroyed by the addition of 7 mL IPA followed by 6 mL 15% NaOH which was sufficent to give a white granular character. The reaction mixture was filtered and the filter cake washed with THF. The filtrate and washings were pooled, stripped of solvent under vacuum providing 3.9 g of a pale amber oil which was dissolved in 250 mL dilute H2SO4. This was washed with 3x75 mL CH2Cl2 which removed the residual yellow color. After making basic with 25% NaOH, the product was extracted with 3x75 mL CH2Cl2 and the solvent removed under vacuum to give 2.72 g of a residue which was distilled at 140-145 °C at 0.2 mm/Hg to give 2.42 g of a clear white oil. This was dissolved in 25 mL IPA, and neutralized with concentrated HCl. This gave a clear solution which, with good stirring, was diluted with 100 anhydrous Et2O to provide 2.40 g 2,5-dimethoxy-4-(i)-propyl-thiophenethylamine hydrochloride (2C-T-4) as white crystals.
DOSAGE: 8 - 20 mg.
DURATION: 12 - 18 h.
QUALITATIVE COMMENTS: (with 8 mg) Visual effects set in at about two hours. There was much color enhancement, particularly of green, and some flowing of colors. The bright impressionistic picture of the little girl, in the bathroom, was particularly good for the visuals to take over, especially when I was concentrating on urinating. The shadows in the large picture above the fireplace would change constantly. I could not either control or turn off these effects during the middle period (3-6 hours). From the physical point of view, something early in the experience simply didn't feel right. Both my lower legs tended to fall asleep, and this seemed to spread to my hands and lower arms. It was uncomfortable and although I was apprehensive at first it didn't get any worse with time so I ignored it. This is not one my favorite materials, and it takes too long to wear off. If I were to do it again I would settle for 4 or 5 milligrams. It may well cut out the extremity problem amd still allow for a pleasant experience.
(with 9 mg) An important characteristic of this experience was the sense of letting go and flowing with it. Just follow where it leads. This seemed to lead to a growing euphoria, a feeling of clearing out of body residues, and the handling of very impressive insights. My thinking continued to grow in clarity, visual perception was crystal clear, and it was a joy to simply look over the scenery, enjoy the beauty, enjoy the companionship, and ponder whatever came to mind. This clarity of body and mind lasted the rest of the evening with a wonderful feeling of peace and centeredness. I still felt a lot of push from the chemical at bed time, causing some tiredness, and allowing very little sleep. I kept working at what had taken place, all night, just to release the experience.
(with 14 mg) Very rational, benign, and good humored. The insight and calm common to the 2C-T's are present, with less of the push of body-energy which makes 2C-T-2 difficult for some people. There are no particular visuals, but then I tend to screen them out consistently, except in cases of mescaline and LSD and psilocybin, so I can't judge what others would experience in the visual area. The eyes-closed imagery is very good without being compelling. The decline is as gradual and gentle as the onset. I am fully capable of making phone calls and other normal stuff. Music is marvelous, and the body feels comfortable throughout.
(with 14 mg) Persistent cold feet, and an uncertain stomach when moving around. Brilliant color trails reminiscent of 2C-B. But a change is occurring and I can't talk myself out of it. There are dark corners. If I were with other people, this would bring out the worst in me, which can be pretty bad.
(with 19 mg) I was caught by the TV. Leonard Bernstein conducting West Side Story. I think I know every note. This was a 1985 rehearsal with the goofs and the sweat. And now Peter, Paul and Mary, grown older along with the songs we all sang. Where Have All the Flowers Gone--and an audience of grown-older people singing Puff the Magic Dragon like earnest children and probably crying along with me. It is good to have lived through the 60's and not to be in them now. Now there's a new song about El Salvador and it's the battle all over again on a different field, but it will always be so, until and unless. Now, in the 80's, I don't get really angry anymore. I am more warrior than angry protester, and that's a much better way to be. In fact, I am quite happy to be where I am. I know a lot more about the game, and what it is, and why it is played, and I have a good idea about my part in it, and I like the part I've chosen.
(with 22 mg) The transition took place over three hours, an alert in 30 minutes followed by a slow and gentle climb. I found it difficult, not physically but mentally since I was for a while locked into the illogical and disconnected aspects of human experiences and expressions, particularly laws and pronouncements and unseeing prejudices, most of which I was picking up from reading the Sunday paper book reviews. As time went on, things became less pushy and I came to be at ease with very positive feelings about everything going on. No self-rejecting aspect at all. Sleep was excellent, but the next day things went slowly and I had to nap a bit. Next time, maybe 18 milligrams.
EXTENSIONS AND COMMENTARY: There are shades of the variability of the Alephs. Some observers are overwhelmed with colors and visual activity; others volunteer their absence. And a very wide range of dosages represented, from an estimated 4 or so milligrams for full effects, to something over 20 milligrams without any loss of control. That is an unusually wide lattitude of activity. And a rich variety of effects that might be experienced. The same wide range of effective dosages was also observed with the corresponding Tweetio. The 2-EtO-homologue of 2C-T-4 is 2-ethoxy-5-methoxy-4-(i)-propylthiophenethylamine, or 2CT4-2ETO. The benzaldehyde (2-ethoxy-5-methoxy-4-(i-propylthio)benzaldehyde had a melting point of 43-44 °C, the nitrostyrene intermediate a melting point of 77-79 °C, and the final hydrochloride a melting point of 153.5-154 °C. There were practically no differences between trials at 5 milligram increments within the 10 and 25 milligram range. Each produced a gentle plus two level of effect which lasted for some 10 hours. A code name of "tenderness" was felt to be appropriate, as there was a peaceful meditative inner receptiveness and clarity noted, with an honest connection felt with those who were present during the experience. Sleep was not comfortable.
I have heard 2C-T-4 referred to as T-4. There is a potent explosive used by terrorists called cyclotrimethylenetrinitramine, known by the code name RDX, or T-4. There is also a T-4 term that refers to thyroxine, an amino acid in the body. The drug 2C-T-4 is neither an explosive nor an amino acid, I am happy to say.
| [ |
[Main Index] | [Forward |

