
#36 2C-P
2,5-DIMETHOXY-4-(n)-PROPYLPHENETHYLAMINE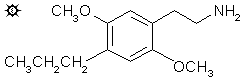
|
| [3D .mol structure] |
A total of 150 g mossy zinc was amalgamated by treatment with a solution of 15 g mercuric chloride in 1 L H2O. After swirling for 0.5 h, the H2O phase was removed by decantation and the zinc added to a 1 L three neck flask. To this there was added 20 mL H2O and 20 mL concentrated HCl, followed by 20 g of 2,5-di-methoxypropiophenone dissolved in 50 mL EtOH. This mixture was held at reflux with a heating mantle overnight, with the occasional addition of HCl as needed to maintain acidic conditions. After cooling to room temperature, the residual solids were removed by filtration, and the filtrate extracted once with 100 mL CH2Cl2 (this was the upper phase). Sufficient H2O was then added to allow extraction with 2x100 mL additional CH2Cl2 with the organic solvent being the lower phase. The combined organic extracts were washed twice with 5% NaOH, followed by one washing with dilute acid. Removal of the solvent under vacuum yielded 18 g of a dark brown oil that was distilled at the water pump to yield 7.2 g of 2,5-dimethoxypropylbenzene as a light yellow oil boiling at 90-130 °C.
A mixture of 22 g 2,5-dimethoxypropylbenzene, 23 g POCl3 and 22 g N-methylformanilide was heated on the steam bath for 1.5 h. The hot, dark reaction mass was poured into 1 L H2O, which allowed the eventual separation of 2,5-dimethoxy-4-(n)-propylbenzaldehyde as a clear yellow oil weighting 14 g. Although the homologous 4-ethyl and 4-butyl benzaldehydes were clean crystalline solids, this propyl homologue remained an oil. Gas chromatographic analysis showed it to be about 90% pure, and it was used as obtained in the nitrostyrene steps with either nitromethane (here) or nitroethane (under DOPR).
To a solution of 13 g 2,5-dimethoxy-4-(n)-propylbenzaldehyde in 100 mL nitromethane, there was added 1.3 g anhydrous ammonium acetate and the mixture held at reflux for 1 h. Removal of the solvent/reactant under vacuum yielded a spontaneously crystallizing mass of orange solids that was removed with the help of a little MeOH. After filtering and air drying there was obtained 7.5 g 2,5-dimethoxy-beta-nitro-4-(n)-propylstyrene with a mp of 118-122 °C. Recrystallization from CH3CN gave an analytical sample with a mp 123-124 °C. Anal. (C13H17NO4) N.
In a 1 L round bottomed flask with a magnetic stirrer under a He atmosphere there was added 120 mL 1 M LAH in tetrahydrofuran. This stirred solution was cooled with an external ice bath, and there was added, dropwise, 3.2 mL of 100% H2SO4, freshly made by the addition of 13.5 g 20% fuming H2SO4 to 15.0 g of ordinary 96% concentrated H2SO4. When the addition was complete, a total of 7.2 g of dry 2,5-dimethoxy-beta-nitro-4-(n)-propylstyrene was introduced as solids in several batches, against a flow of He, over the course of 20 min. The reaction mixture was allowed to come to room temperature, and stirred for an additional 0.5 h, then brought to reflux for 10 min on the steam bath. The excess hydride was destroyed with 18 mL IPA, and then sufficient 15% NaOH was added which made the aluminum oxides distinctly basic and of a filterable texture. The inorganics were removed by filtration, and the filter cake washed with additional THF. The combined filrate and washes were stripped of solvent, yielding several g of a pale yellow oil that was suspended in a large quantity of dilute H2SO4. The aqueous phase was filtered free of insolubles, washed with a little CH2Cl2, and made basic with aqueous NaOH. This was extracted with 3x40 mL CH2Cl2 and, after the removal of the solvent under vacuum, the residual 2 g of off-white oil was distilled. A fraction that distilled at 100-110 °C at 0.3 mm/Hg was water white, weighed 1.59 g and spontaneously crystallized. This fraction was dissolved in 7.5 mL warm IPA and neutralized with 0.6 mL concentrated HCl. The spontaneous crystals of 2,5-di-methoxy-4-(n)-propylphenethylamine hydrochloride (2C-P) were suspended in 20 mL anhydrous Et2O, filtered, Et2O washed, and air dried. The weight was 1.65 g and the mp was 207-209 °C with prior sintering at 183 °C., Anal. (C13H22ClNO2) N.
DOSAGE: 6 - 10 mg.
DURATION: 10 - 16 h.
QUALITATIVE COMMENTS: (with 6 mg) I was not feeling so good. Hangover, I guess. The material was so gentle in coming on, and soon my body became jangled. Thinking was easy. Verbalizing was easy. Being comfortable with my body was not. My back hurt and then my legs hurt. My lower back was in spasm. At first I did not particularly like what this drug was doing to my body, but took a good look at it and decided that I was the culprit. Took a good look at my drinking so much, and decided that I didn't need it. So much energy was going through me I didn't know what to do with it. The whole day was spent in physical discomfort. Food tasted good, and we nibbled all day. My stomach was bloated. Next day I was more or less like a zombie. I was wiped out.
(with 8 mg) Comes on slowly, not feeling intently until into 2nd hour. I feel slight discomfort but override it responding to music. I take in air, directing it inside to heal uncomfortable places, open up my clogged sinuses. Wonderful experience of clean, fresh, healing air. Find that discomfort zone is places where I think there is something wrong with me. I dissolve these places with the feeling I'm OK. Like myself better and better, and find more reasons to enjoy and appreciate myself. I find this material powerful, and an excellent working material. Under other circumstances, would probably spend more time working alone inside, where there were great openings, and some of the most beautiful visuals I have seen for a long time. Usually I do not get visuals. I like the long action. I feel that this material worked for a good week after the experience, with internal processes taking place, many insights, and energy running. At times the energy was a little uncom-fortable, but could always be quelled by taking a moment for deep relaxation or looking directly at the internal process. I feel that much good internal work has been done, a lot of it unconscious.
(with 9 mg) At the one hour point, I am barely off of baseline. It is not until almost the third hour that the experience is fully developed, and once there it is maintained for another four hours. I was well grounded but rather diffuse. I explored writing (which went quite well), interpretation (pictures and reading both OK) and talking (very good). This is an excellent level, and probably near the max.
(with 12 mg) Slow and even rise. At five minutes to seven (suddenly the clock time makes no sense at all) I am at a 3+ and feel that I have not yet plateau'd. Erotic was excellent. Music good. Eyes-closed imagery very different place than usual experiences. Slow, calm, strong images from an area that has no apparent connection with usual waking world, yet underlies all of it. A cool, wise place which has its own rules. All emotions and feeling available, but there is a cool perspective which informs all thinking. Talking superb and fun, and it was possible to feel our bodies healthy and full of determination to remain so, despite obvious faults and self-indulgences. Could do a lot of learning with this material, but probably not a group thing. It would lend itself too easily to hypnotic power-games, and it would be too easy to open up the shared consciousness level, which would be frightening to a lot of people and bring about necessary escapes such as sickness. Excellent feeling the next day.
EXTENSIONS AND COMMENTARY: There is certainly a broad mixture of experiences with 2C-P but, on the whole, probably more favorable than not. There was one report of an experience in which a single dosage of 16 mg was clearly an overdose, with the entire experiment labeled a physical disaster, not to be repeated. A consistent observation is that there may not be too much latitude in dosage between that which would be modest, or adequate, and that which would be excessive. The need for individual titration would be most important with this compound.
| [ |
[Main Index] | [Forward |

