
#16 BOHD
2,5-DIMETHOXY-beta-HYDROXY-4-METHYLPHENETHYLAMINE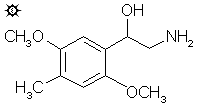
|
| [3D .mol structure] |
DOSAGE: greater than 50 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 50 mg) At about the two hour point, there was a precipitous drop of blood pressure (from 120/72 to 84/68) although the pulse stayed steady at 60. This trend had been apparent in earlier trials, and was being watched carefully. No further tests are planned.
EXTENSIONS AND COMMENTARY: The usual method of making beta-ethanolamine such as this is through the reduction of the cyanohydrin of the corresponding benzaldehyde and, in fact, that method is described in the recipe for DME. This above procedure was actually part of an exploration of different agents that might be used in the reduction of the intermediate nitroalkane. This product was the unexpected result of trying zinc.
Why the potent cardiovascular effect seen by this compound? There are a couple of points that might argue for some adrenolytic toxicity. This material is a beta-ethanolamine and, with maybe one or two exceptions, clinically used beta-receptor blockers are beta-ethanolamines. In fact, a few of these so-called beta-blockers actually have two methoxy groups on the aromatic rings, also a property of BOHD. The antidiabetic drug Butaxamine (BW 64-9 in the code of Burroughs Wellcome) is identical to BOHD except that the 4-methyl group is on the alpha-carbon instead, and there is a tertiary butyl group on the nitrogen atom. Another point involves the proximity of the beta-hydroxy group and the methoxyl oxygen atom in the 2-position of the ring. There is going to be a strong hydrogen-bonding with this orientation, with the formation of a stable six-membered ring. This might help obscure the hydrophilic nature of the free hydroxyl group and allow the compound to pass into the brain easily. If this group is masked by an easily removed group such as an acetate ester, one gets the compound beta-acetoxy-3,4-dimethoxy-4-methylphenethylamine (BOAD) which is similar to BOHD as a hypotensive.
The code-naming procedure used here (and elsewhere here in Book II) is: (1) to use RBOS as the alert to there being an oxygen on the benzyl carbon of a phenethylamine (it is a benzyl alcohol); (2) if there is just one more letter (a third and last letter) it will identify the 2C-X parent from which it has been derived [RBS comes from 2C-B, RDS comes from 2C-D, RHS comes from homopiperonylamine (MDPEA) rather than from 2C-H, RMS comes from mescaline, and in every case the beta-substituent is a methoxy group]; and (3) if there are four letters, then the fourth letter is as above, and the third letter (the next to last letter) is the substituent on that benzylic oxygen. With a three letter code, the substituent is a methyl group, an RHS for a third letter of four makes it a hydroxyl group, and an RAS for the third letter is an acetyl group, and an RES is for an ethyl group. A similar sort of cryptographic music was composed by Du Pont in their three-number codes for the Freons. The first number was one less than the number of carbons in the molecule, the second number was one more than the number of hydrogens in the molecule, the third number was the exact number of fluorines in the molecule, and the rest of the bonds were filled with chlorines, Thus Freon 11 (really Freon 011) was trichlorofluoromethane and Freon 116 was hexafluoroethane.
Complex, yes. But both systems are completely straightforward, and flexible for future creations. A few additional examples of similar beta-ethanolamines are scattered throughout Book II and they have, in general, proved to be uninteresting, at least as potential psychedelic compounds.
| [ |
[Main Index] | [Forward |

