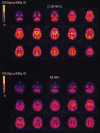
Positron emission tomographic evidence of toxic effect of MDMA ("Ecstasy")
on brain serotonin neurons in human beings
Published in The Lancet
Volume 352, Number 9138 31 October 1998
All Copyrights http://www.thelancet.com/
U D McCann, Z Szabo, U Scheffel, R F Dannals, G A Ricaurte
Biological Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland, USA (U D McCann MD); and Departments of Radiology (Z Szabo MD, U Scheffel ScD, R F Dannals PhD) and Neurology (G A Ricaurte MD), Johns Hopkins Medical Institutions, Baltimore, MD 21224, USA
Correspondence to: Dr G A Ricaurte
 Summary
Summary
 Introduction
Introduction
 Methods
Methods
 Results
Results


 Discussion
Discussion
 References
References

Methods We enrolled 14 previous users of MDMA who were currently abstaining from use and 15 controls who had never used MDMA. We used positron emission tomography (PET) with the radioligand carbon-11-labelled McN-5652, which selectively labels the 5-HT transporter. We analysed whether there were differences in 5-HT transporter binding between abstinent MDMA users and participants in the control group. Blood and urine samples were taken and tested to check for abstinence.
Findings MDMA users showed decreased global and regional brain 5-HT transporter binding compared with controls. Decreases in 5-HT transporter binding positively correlated with the extent of previous MDMA use.
Interpretation Quantitative PET studies with a ligand selective for 5-HT transporters can be used to assess the status of 5-HT neurons in the living human brain. We show direct evidence of a decrease in a structural component of brain 5-HT neurons in human MDMA users.
Lancet 1998; 352: 1433-37

Previous studies of human MDMA users have used indirect markers of brain 5-HT neurons, such as cerebrospinal fluid concentrations of 5-hydroxyindoleacetic acid, to show possible brain 5-HT axonal injury.15,16 These studies have found selective decreases of 5-hydroxyindoleacetic acid in cerebrospinal fluid of MDMA-exposed human beings similar to those seen in animals with documented 5-HT axonal injury.8,17 Human MDMA users may, therefore, also be susceptible to MDMA-induced 5-HT neurotoxic effects. Many factors influence cerebrospinal fluid concentrations of 5-hydroxyindoleacetic acid,18 however, and additional evidence is required before conclusions can be reached about the presence and consequences of MDMA-induced brain 5-HT neurotoxic effects in human beings.
Advances in neuroimaging techniques such as positron emission tomography (PET) have made it possible to assess the status of chemically-defined populations of neurons in the living human brain. For example, development of carbon-11-labelled McN-5652, a radioligand that selectively labels the 5-HT transporter,19-22 has made it possible to directly and quantitatively assess the status of brain 5-HT neurons in living human beings. The specific binding of [11C]McN-5652 parallels the known distribution of 5-HT-transporter sites in the human brain, and binding is completely blocked by pretreatment with a selective 5-HT reuptake inhibitor.22 The 5-HT transporter is a structural element of the 5-HT neuron that is substantially decreased in animals given neurotoxic doses of MDMA and related drugs.1-9 Moreover, PET imaging with [11C]McN-5652 has been validated as a technique for detecting substituted amphetamine-induced 5-HT injury in baboons.23-25
We investigated specific [11C]McN-5652 binding to find out whether MDMA use leads to a lasting quantitative alteration in [11C]McN-5652-labelled 5-HT-transporter sites in the human brain.

We screened serum and urine samples with an enzyme multiplied immunoassay (EMIT, Behring Diagnostics Inc, San Jose, CA, USA) for amphetamines, barbiturates, benzodiazepine metabolites, cocaine and metabolite, opiates, phencyclidine, and marijuana. Some drugs, depending on degree of use, may be detected 2-3 weeks after use (eg, marijuana). Amphetamines (including MDMA) could be detected only 24-48 h after the last dose. When necessary, we assessed serum samples with gas chromatography or mass spectroscopy by standard diagnostic laboratory methods.
On a magnetic-resonance scanner, we obtained two sequences--one for identification of the anterior commissural-posterior commissural line, and another for coregistration with PET. The first sequence consisted of a set of T1-weighted scout images with the following imaging variables: TR-500, TE-20, slice thickness 5 mm with no gap, 128×256 matrix, and 1 nex. The second sequence was an axial spin density/T1-weighted, 3D volumetric scan using rf "spoiled" gradient echoes. This dataset was used for identification of grey matter and white matter. Imaging variables were: TR 35, TE 5, flip angle 45O, 1·5 mm effective slice thickness, no gap, 124 slices within plane 192×256 matrix, 24 cm field of view, and 1 nex.
For PET scanning, we used a whole-body scanner that acquires 15 simultaneous slices, spaced 6·5 mm apart. The scanner had a transaxial (in-plane) resolution of 5·4 mm and an average axial resolution of 6 mm (full width at half maximum), measured in the centre of the field of view. PET scans were performed in two dimensional mode. Scatter correction was done by subtraction of scatter, based on a measured scatter-response function.27 Participants were positioned in the scanner in accordance with the previously drawn coregistration line. A transmission scan was acquired for 10 min with 370 MBq germanium-68/gallium-68 pin source. We took two PET scans for each participant, one with [11C](+)McN-5652, and another with [11C](-)McN-5652, with 150 min between injections. The injected dose at time of injection was 684·5 (SD 44·4) MBq for both [11C](+)McN-5652 and [11C](-)McN-5652 (specific activity 78292 [7067] MBq/µmol and 79476 [5661]) MBq/µmol, respectively). 18 serial scans were obtained in dynamic mode during 95 min with the image sequence: 4×15 s, 3×1 min, 3×2 min, 3×5 min, 3×10 min, and 2×20 min.
Positioning was closely monitored during acquisition, and any deviation from the original line was corrected. The PET scans were decay-corrected and reconstructed with ramp-filtered back-projection in a 128×128 matrix, with a pixel size of 2×2 mm, and were attenuation-corrected with the transmission scan. The scans were transferred to a computer for image analysis with software developed inhouse (JHU Imager-3D).
Arterial blood samples were obtained every 3-7 s during the first 2-3 min after ligand injection, and at times with increasing intervals until 95 min after injection. We analysed the arterial plasma samples with high-performance liquid chromatography, and we corrected the input function for metabolised radioligand activity. High-performance liquid chromatography was done after loading the plasma on solid-phase extraction cartridge (Millipore Inc, Bedford, MA, USA) columns and extracting the radioactivity by methanol. A high performance liquid chromatograph column (Econosil, Altech, Deerfield, IL, USA) was used as the solid phase, with 50% acetonitrile and 50% water buffered with 0·1 mmol/L ammonium formate as the mobile phase for high-performance liquid chromatography. Measured radioactivity was digitally stored and analysed on computer (Dynamax).
Region-of-interest placement was based on coregistered magnetic-resonance and PET images. We coregistered PET and magnetic-resonance scans with three fiducials visible on each scan. Magnetic-resonance images were registered to PET images by linear transformation. Regions of interest were drawn on the PET images by an investigator unaware of the participant's history. Regions of interest were the frontal cortex, parietal cortex, temporal cortex, occipital and cingulate cortex, caudate, putamen, thalamus, midbrain, pons, hypothalamus, and cerebellum. The average size of these regions was 44 pixels.
To analyse radioligand binding, we tested four different compartmental models before identifying a one-tissue compartment, three-variable model28,29 that provides the most robust kinetic variables. The most complex model tested had two tissue compartments: one for specific binding of the radioligand and one for everything else (non-specific binding and free ligand). This model was tested in two versions: one with and one without substantial contribution from cerebral blood activity, designated as BV. In addition to BV, the kinetic variables were K1 and k2, which are used to describe uptake and release in the brain, and k3 and k4, which describe binding and release at the specific binding sites. This model was also tested with the four kinetic variables but without BV. We used ANOVA to compare models; we calculated F values from the sums of squared residuals of the curve fits.30 In the one-tissue compartment model selected, the specific and non-specific binding compartments become one compartment, and two (K1, k2) or three (BV, K1, k2) variables are estimated to describe radioligand kinetics. Model variables were estimated by keeping to a minimum the summed squared difference between actual tissue activity measured with PET and estimated tissue activity by the Marquardt technique.31 Time-activity curves were synthesised from the set of differential equations by the fourth-order Runge-Kutta technique.32
In the one-tissue compartment, three-parameter model that we used, the DV is obtained from the ratio of uptake (K1) and release (k2) variables; therefore, DV=K1/k2. The assumption was made that the tissue DV of [11C](+)McN-5652 consisted of three components: specific binding, non-specific binding, and free ligand, whereas the DV of [11C](-)McN-5652 consisted of only non-specific binding and free ligand.
We used logarithmic transforms of DVs corrected for non-specific binding to achieve a normal distribution in the control and MDMA groups, and to enable analysis of variance. Logarithmic transformation across all regions in participants in the control group resulted in a pooled coefficient of variation of 22%.

In the MDMA group, participants had generally used MDMA on more than 200 occasions and over 4-5 years. Most of the participants had not used MDMA for months, and some had not used MDMA for several years (table 2).
The injected doses of radioligands did not differ significantly in the two groups (table 3). We plotted representative time-activity curves with model fits for [11C](+)McN-5652 and [11C](-)McN-5652 for the two groups (figure 1). Although control and MDMA participants had similar transport of [11C]McN-5652 from blood to brain (K1) for all brain regions assessed, MDMA users had significant global decreases in DVs for specific binding of [11C]McN-5652 (p=0·011), which suggests that they had a lower density of brain 5-HT transporter sites than participants in the control group (figure 2). The covariance effects of age and sex were not significant (p=0·95 and p=0·16, respectively). Decreases of regional brain DVs in human MDMA users (figure 3) are consistent with PET findings in MDMA-treated baboons.23
Decreases in 5-HT transporter binding positively correlated with extent of previous MDMA use, (r=-0·50, p=0·005, figure 4), consistent with data in animals of exposure-related loss of 5-HT terminals after MDMA administration.1,2,5-8 There was no correlation between the duration of abstinence from MDMA and the extent of specific [11C]McN-5652 binding (r=-0·09, p=0·75). All participants in the MDMA group were negative for recent drug use.



All participants in the MDMA group in our study reported that they had abstained from use of MDMA or other psychoactive drugs for at least 3 weeks before the study, which suggests that the decreases we saw in brain [11C]McN-5652-labelled 5-HT-transporter sites were not due to pharmacological effects of MDMA or other drugs. Our results do not, however, rule out the possibility that decreased 5-HT-transporter binding sites are secondary to pre-existing differences in 5-HT function in MDMA users compared with controls, but since none of the MDMA users had a neuropsychiatric disorder in which 5-HT has been implicated, this possibility is unlikely. Finally, although most of the MDMA users had experimented with other recreational drugs, none was a known 5-HT neurotoxin in human beings, and was not likely to account for changes in 5-HT binding.
Our data do not allow conclusions about reversibility or permanence of MDMA-induced changes in brain 5-HT transporter. Although we found no correlation between the duration of abstinence and the extent of decrease in [11C]McN-5652 binding, MDMA-induced changes may be reversible. Sample sizes and various other factors could have contributed to the apparent absence of recovery. More MDMA users with varied durations of abstinence and drug exposure histories must be studied to show whether 5-HT-terminal structure and function return to normal over time. Studies in non-human primates show that MDMA-induced changes in 5-HT terminal markers persist for longer than 1 year after doses of MDMA similar to those used by some human MDMA users.10,11,14
Our finding that a selective 5-HT transporter ligand can be used to show the pathophysiological state of 5-HT neurons in living human brains might have broad implications for many neuropsychiatric illnesses in which brain 5-HT neurons have been implicated, including depression, anxiety, and cognitive dysfunction.33,34 Quantitative PET imaging studies with [11C]McN-5652 should help to define the role of alterations in the 5-HT transporter in the basic pathophysiology of 5-HT-linked neuropsychiatric disorders and their response to treatment with selective 5-HT reuptake inhibitors.
A potential drawback of our study is that we relied upon participants' reports of drug use and duration of abstinence. This is an inherent problem in all studies of illicit drug use. Our participants are unlikely, however, to have altered their drug-use history because they contacted us and identified themselves as MDMA users. Drug tests indicated that no participant used marijuana in the 2-3 weeks before the study, despite several participants being regular marijuana users. Participants were told that they would undergo drug testing at the time of admission to the study, and that they would not receive payment if they had positive results for psychoactive drugs. Knowledge of drug testing upon arrival was probably a good deterrent from drug use, and because participants underwent PET scans 48 h after drug testing, we can be certain that participants abstained from amphetamines for 4 days before PET imaging. In future studies, hair-sample analysis would be a more useful way to ascertain long periods of abstinence from MDMA.
Sex differences in the effects of MDMA exposure on [11C]McN-5652 binding should be studied further. Our analysis showed no differences between men and women in the two groups. Nevertheless, the sample was small, and differences might be found if more women were studied. No sex differences in susceptibility to MDMA neurotoxic effects have been found in animals, but additional studies with participants matched for age and sex are necessary.
In summary, our data suggest that people who use MDMA as a recreational drug are unwittingly putting themselves at risk of developing brain 5-HT neural injury. In addition, systematic studies of MDMA-exposed individuals with highly selective brain 5-HT transporter deficits may give important insights into the functional role of brain 5-HT in human behaviour. Potential functional consequences of MDMA-induced brain 5-HT neurotoxic lesions are not yet clear, but may include depression, anxiety, memory disturbance, and other neuropsychiatric disorders in which brain 5-HT has been implicated.33,34

2 Commins DL, Vosmer G, Virus R, Woolverton W, Schuster C, Seiden L. Biochemical and histological evidence that methylenedioxymethamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther 1987; 241: 338-45.
3 Battaglia G, Yeh SY, O'Hearn E, Molliver ME, Kuhar MJ, DeSouza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labelled serotonin uptake sites. J Pharmacol Exp Ther 1987; 242: 911-16.
4 O'Hearn EG, Battaglia G, DeSouza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci 1988; 8: 2788-803.
5 Slikker W, Ali SF, Scallet C, Frith CH, Newport GD, Bailey JR. Neurochemical and neurohistological alterations in the rat and monkey produced by orally administered methylenedioxymethamphetamine (MDMA). Toxicol Appl Pharmacol 1988; 94: 448-57.
6 Ricaurte GA, Forno LS, Wilson MA, et al. (±)3,4-methylenedioxymethamphetamine (MDMA) selectively damages central serotonergic neurons in non-human primates. JAMA 1988; 260: 51-55.
7 Ricaurte GA, DeLanney LE, Irwin I, Langston JW. Toxic effects of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration. Brain Res 1988; 446: 165-68.
8 Insel TR, Battaglia G, Johannessen JN, Marra S, De Souza EB. 3,4-Methylenedioxymethamphetamine ("ecstasy") selectively destroys brain serotonin terminals in rhesus monkeys. J Pharmacol Exp Ther 1989; 249: 713-20.
9 Molliver ME, Berger UV, Mamounas LA, Molliver DC, O'Hearn EG, Wilson MA. Neurotoxicity of MDMA and related compounds: anatomic studies. Ann NY Acad Sci 1990; 600: 640-64.
10 Ricaurte GA, Martello AL, Katz JL, Martello MB. Lasting effects of (±)3,4-methylenedioxymethamphetamine on central serotonergic neurons in non-human primates: neurochemical observations. J Pharmacol Exp Ther 1992; 261: 616-22.
11 Fischer CA, Hatzidimitriou G, Katz JL, Ricaurte GA. Reorganization of ascending serotonin axon projections in animals previously exposed to the recreational drug 3,4-methylenedioxymethamphetamine. J Neurosci 1995; 15: 5476-85.
12 Ricaurte GA. Studies of MDMA-induced neurotoxicity in nonhuman primates: a basis for evaluating long-term effects in humans. In: Asghar K, DeSouza E, eds. Pharmacology and toxicology of amphetamines and related designer drugs. NIDA Res Monogr 1989; 94: 306-22.
13 Eisner B. Ecstasy: the MDMA Story. Berkeley: Ronin Publishing, 1989.
14 Chappell W, Mordenti J. Extrapolation of toxicological and pharmacological data from animals to humans. In: Tests B, ed. Advances in drug research. San Diego: Academic Press, 1991: 1-116.
15 Ricaurte GA, Finnegan KF, Irwin I, Langston JW. Aminergic metabolites in cerebrospinal fluid of humans previously exposed to MDMA: preliminary observations. Ann NY Acad Sci 1990; 600: 699-708.
16 McCann UD, Ridenour A, Shaham Y, Ricaurte GA. Serotonin neurotoxicity after MDMA: a controlled study in humans. Neuropsychopharmacology 1994; 10: 129-38.
17 Ricaurte GA, DeLanney LE, Wiener SG, Irwin I, Langston JW. 5-Hydroxyindoleacetic acid in cerebrospinal fluid reflects serotonergic damage induced by 3,4-methylenedioxymethamphetamine in CNS of non-human primates. Brain Res 1989; 474: 359-63.
18 Brewerton TD, Berrettini WH, Nurnberger JI, Linnoila M. Analysis of seasonal fluctuations of CSF monoamine metabolites and neuropeptides in normal controls: findings with 5-HIAA and HVA. Psychiatr Res 1989; 23: 257-65.
19 Dannals RF, Scheffel U, Suehiro M, Ricaurte G. Radioligand development for studying serotonin uptake sites with positron emission tomography. Med Chem Res 1994; 5: 228-44.
20 Suehiro M, Scheffel U, Dannals RF, Ricaurte GA, Ravert HT, Wagner HN. A PET radiotracer for studying serotonin uptake sites: [11C]McN-5652-Z. J Nucl Med 1993; 34: 120-27.
21 Szabo Z, Sceffel U, Suehiro M, et al. Positron emission tomography of serotonin transporter sites in baboon brain with [11C]McN5652. J Cereb Blood Flow Metab 1995; 15: 798-805.
22 Szabo Z, Kao P, Scheffel U, et al. Positron emission tomography of serotonin transporter sites in human brain with [11C]McN5652. Synapse 1995; 20: 37-43.
23 Scheffel S, Szabo Z, Matthews W, Dannals R, Ravert H, Ricaurte G. Fenfluramine-induced loss of serotonin transporters in baboon brain visualized with PET. Synapse 1996; 24: 395-98.
24 Scheffel U, Szabo Z, Mathews WB, et al. In vivo detection of short- and long-term MDMA neurotoxicity: a positron emission tomography study in the living baboon brain. Synapse 1998; 29: 183-92.
25 Scheffel U, Szabo Z, Mathews W, et al. Validation of compartmental and noncompartmental kinetic models for [11C]McN5652 binding in baboons. Soc Neurosci Abstracts 1997; 23: 558.
26 Suehiro M, Ravert HT, Dannals RF, et al. Synthesis of a radiotracer for studying 5-HT uptake sites with positron emission tomography: [11C]McN-5652-Z. J Lab Comp Radiopharm 1992; 31: 841-48.
27 Bergström M, Eriksson L, Bohm C, Blomqvist G, Litton J. Correction for scattered radiation in a ring detector positron camera by integral transformation of the projections. J Comput Assist Tomogr 1983; 7: 42-50.
28 Frey KA, Koeppe RA, Kilbourne MR, et al. Presynaptic monoaminergic vesicles in Parkinson's disease and normal aging. Ann Neurol 1996; 40: 873-84.
29 Koeppe RA, Frey KA, Van der Borght TM, et al. Kinetic evaluation of [11C]dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab 1996; 16: 1288-99.
30 Muzik O, Chugani D, Chakraborty O, et al. Analysis of C-11 alpha-methyl-tryptophan kinetics for estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab 1997; 17: 659-69.
31 Marquardt DW. An algorithm for least-squares estimation of nonlinear parameters. J Soc Industr Appl Math 1963; 11: 431-41.
32 Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical recipes. In: The art of scientific computing. Cambridge, USA: University Press, 1986.
33 Coccaro EF, Murphy DL. Serotonin in Major Psychiatric Disorders. Washington, DC: American Psychiatric Press, 1990.
34 Sandler M, Coppen A, Harnett S. 5-Hydroxytryptamine in psychiatry: a spectrum of ideas. Oxford: Oxford University Press, 1991.
Biological Psychiatry Branch, National Institute of Mental Health, Bethesda, Maryland, USA (U D McCann MD); and Departments of Radiology (Z Szabo MD, U Scheffel ScD, R F Dannals PhD) and Neurology (G A Ricaurte MD), Johns Hopkins Medical Institutions, Baltimore, MD 21224, USA
Correspondence to: Dr G A Ricaurte
Summary
Background (±)3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") is a popular recreational drug that selectively damages brain serotonin (5-HT) neurons in animals at doses that closely approach those used by humans. We investigated the status of brain 5-HT neurons in MDMA users.Methods We enrolled 14 previous users of MDMA who were currently abstaining from use and 15 controls who had never used MDMA. We used positron emission tomography (PET) with the radioligand carbon-11-labelled McN-5652, which selectively labels the 5-HT transporter. We analysed whether there were differences in 5-HT transporter binding between abstinent MDMA users and participants in the control group. Blood and urine samples were taken and tested to check for abstinence.
Findings MDMA users showed decreased global and regional brain 5-HT transporter binding compared with controls. Decreases in 5-HT transporter binding positively correlated with the extent of previous MDMA use.
Interpretation Quantitative PET studies with a ligand selective for 5-HT transporters can be used to assess the status of 5-HT neurons in the living human brain. We show direct evidence of a decrease in a structural component of brain 5-HT neurons in human MDMA users.
Lancet 1998; 352: 1433-37
Introduction
Findings in animals (including non-human primates) have suggested that the popular recreational drug (±)3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") might damage brain 5-HT neurons in human beings. Animals given MDMA develop persistent losses in various markers unique to 5-HT axons, including 5-HT, 5-hydroxyindoleacetic acid, tryptophan hydroxylase, and the 5-HT transporter,1-9 which suggests a distal axotomy of central 5-HT neurons. In primates, the loss of 5-HT axonal markers is long-lasting7-8 and may, in some brain regions, be permanent.10,11 Doses of MDMA that produce neurotoxic effects in non-human primates7,12 overlap with doses used recreationally by some human beings,13 especially when differences in body mass and surface area are taken into account by interspecies scaling methods.14Previous studies of human MDMA users have used indirect markers of brain 5-HT neurons, such as cerebrospinal fluid concentrations of 5-hydroxyindoleacetic acid, to show possible brain 5-HT axonal injury.15,16 These studies have found selective decreases of 5-hydroxyindoleacetic acid in cerebrospinal fluid of MDMA-exposed human beings similar to those seen in animals with documented 5-HT axonal injury.8,17 Human MDMA users may, therefore, also be susceptible to MDMA-induced 5-HT neurotoxic effects. Many factors influence cerebrospinal fluid concentrations of 5-hydroxyindoleacetic acid,18 however, and additional evidence is required before conclusions can be reached about the presence and consequences of MDMA-induced brain 5-HT neurotoxic effects in human beings.
Advances in neuroimaging techniques such as positron emission tomography (PET) have made it possible to assess the status of chemically-defined populations of neurons in the living human brain. For example, development of carbon-11-labelled McN-5652, a radioligand that selectively labels the 5-HT transporter,19-22 has made it possible to directly and quantitatively assess the status of brain 5-HT neurons in living human beings. The specific binding of [11C]McN-5652 parallels the known distribution of 5-HT-transporter sites in the human brain, and binding is completely blocked by pretreatment with a selective 5-HT reuptake inhibitor.22 The 5-HT transporter is a structural element of the 5-HT neuron that is substantially decreased in animals given neurotoxic doses of MDMA and related drugs.1-9 Moreover, PET imaging with [11C]McN-5652 has been validated as a technique for detecting substituted amphetamine-induced 5-HT injury in baboons.23-25
We investigated specific [11C]McN-5652 binding to find out whether MDMA use leads to a lasting quantitative alteration in [11C]McN-5652-labelled 5-HT-transporter sites in the human brain.
Methods
Participants
We enrolled nine men and five women who reported previous heavy use of MDMA. Recruitment was through advertisements (local newspapers and worldwide web) and referrals. Participants agreed to abstain from use of psychoactive drugs for at least 3 weeks before the study, and were asked to undergo drug screening before enrolment. We did structured diagnostic psychiatric interviews with Scheduled Interview for Diagnostic and Statistical Manual of Mental Disorders IV (SCID-IV) to confirm the absence of any current axis I psychiatric diagnoses in which 5-HT has been implicated. The control group consisted of nine men and six women who had no previous experience with MDMA and were free of axis I psychiatric diagnoses. The eligibility criterion for the MDMA group was previous use of MDMA on at least 25 occasions. After testing of blood and urine samples, exclusion criteria were: a positive drug screen; pregnancy; a severe medical or neuropsychiatric illness that precluded informed consent; claustrophobia or a cardiac pacemaker or surgical clip; and a neuropsychiatric disease in which 5-HT has been implicated. Participants were paid for taking part in the study. We obtained written informed consent from all participants. The study was approved by the institutional review board.Drug screening
We obtained information about MDMA and other drug use by a preliminary telephone interview, followed by a questionnaire that asked specifically about MDMA use, a drug-history questionnaire (drug history section of the addiction severity index), and relevant portions of the SCID-IV.We screened serum and urine samples with an enzyme multiplied immunoassay (EMIT, Behring Diagnostics Inc, San Jose, CA, USA) for amphetamines, barbiturates, benzodiazepine metabolites, cocaine and metabolite, opiates, phencyclidine, and marijuana. Some drugs, depending on degree of use, may be detected 2-3 weeks after use (eg, marijuana). Amphetamines (including MDMA) could be detected only 24-48 h after the last dose. When necessary, we assessed serum samples with gas chromatography or mass spectroscopy by standard diagnostic laboratory methods.
Imaging
We synthesised [11C](+)McN-5652 and [11C](-)McN-5652 with a previously described method.26 Before imaging, thermoplastic face masks were made for each participant to their individual measurements. These could be attached firmly to head holders in PET and magnetic-resonance scanners. We used a slice through the mid-sagittal plane of the brain scanned with magnetic-resonance imaging to identify the anterior commissural-posterior commissural line. We labelled the intersection of this plane on the mask as a reference for further scanning.On a magnetic-resonance scanner, we obtained two sequences--one for identification of the anterior commissural-posterior commissural line, and another for coregistration with PET. The first sequence consisted of a set of T1-weighted scout images with the following imaging variables: TR-500, TE-20, slice thickness 5 mm with no gap, 128×256 matrix, and 1 nex. The second sequence was an axial spin density/T1-weighted, 3D volumetric scan using rf "spoiled" gradient echoes. This dataset was used for identification of grey matter and white matter. Imaging variables were: TR 35, TE 5, flip angle 45O, 1·5 mm effective slice thickness, no gap, 124 slices within plane 192×256 matrix, 24 cm field of view, and 1 nex.
For PET scanning, we used a whole-body scanner that acquires 15 simultaneous slices, spaced 6·5 mm apart. The scanner had a transaxial (in-plane) resolution of 5·4 mm and an average axial resolution of 6 mm (full width at half maximum), measured in the centre of the field of view. PET scans were performed in two dimensional mode. Scatter correction was done by subtraction of scatter, based on a measured scatter-response function.27 Participants were positioned in the scanner in accordance with the previously drawn coregistration line. A transmission scan was acquired for 10 min with 370 MBq germanium-68/gallium-68 pin source. We took two PET scans for each participant, one with [11C](+)McN-5652, and another with [11C](-)McN-5652, with 150 min between injections. The injected dose at time of injection was 684·5 (SD 44·4) MBq for both [11C](+)McN-5652 and [11C](-)McN-5652 (specific activity 78292 [7067] MBq/µmol and 79476 [5661]) MBq/µmol, respectively). 18 serial scans were obtained in dynamic mode during 95 min with the image sequence: 4×15 s, 3×1 min, 3×2 min, 3×5 min, 3×10 min, and 2×20 min.
Positioning was closely monitored during acquisition, and any deviation from the original line was corrected. The PET scans were decay-corrected and reconstructed with ramp-filtered back-projection in a 128×128 matrix, with a pixel size of 2×2 mm, and were attenuation-corrected with the transmission scan. The scans were transferred to a computer for image analysis with software developed inhouse (JHU Imager-3D).
Arterial blood samples were obtained every 3-7 s during the first 2-3 min after ligand injection, and at times with increasing intervals until 95 min after injection. We analysed the arterial plasma samples with high-performance liquid chromatography, and we corrected the input function for metabolised radioligand activity. High-performance liquid chromatography was done after loading the plasma on solid-phase extraction cartridge (Millipore Inc, Bedford, MA, USA) columns and extracting the radioactivity by methanol. A high performance liquid chromatograph column (Econosil, Altech, Deerfield, IL, USA) was used as the solid phase, with 50% acetonitrile and 50% water buffered with 0·1 mmol/L ammonium formate as the mobile phase for high-performance liquid chromatography. Measured radioactivity was digitally stored and analysed on computer (Dynamax).
Region-of-interest placement was based on coregistered magnetic-resonance and PET images. We coregistered PET and magnetic-resonance scans with three fiducials visible on each scan. Magnetic-resonance images were registered to PET images by linear transformation. Regions of interest were drawn on the PET images by an investigator unaware of the participant's history. Regions of interest were the frontal cortex, parietal cortex, temporal cortex, occipital and cingulate cortex, caudate, putamen, thalamus, midbrain, pons, hypothalamus, and cerebellum. The average size of these regions was 44 pixels.
To analyse radioligand binding, we tested four different compartmental models before identifying a one-tissue compartment, three-variable model28,29 that provides the most robust kinetic variables. The most complex model tested had two tissue compartments: one for specific binding of the radioligand and one for everything else (non-specific binding and free ligand). This model was tested in two versions: one with and one without substantial contribution from cerebral blood activity, designated as BV. In addition to BV, the kinetic variables were K1 and k2, which are used to describe uptake and release in the brain, and k3 and k4, which describe binding and release at the specific binding sites. This model was also tested with the four kinetic variables but without BV. We used ANOVA to compare models; we calculated F values from the sums of squared residuals of the curve fits.30 In the one-tissue compartment model selected, the specific and non-specific binding compartments become one compartment, and two (K1, k2) or three (BV, K1, k2) variables are estimated to describe radioligand kinetics. Model variables were estimated by keeping to a minimum the summed squared difference between actual tissue activity measured with PET and estimated tissue activity by the Marquardt technique.31 Time-activity curves were synthesised from the set of differential equations by the fourth-order Runge-Kutta technique.32
In the one-tissue compartment, three-parameter model that we used, the DV is obtained from the ratio of uptake (K1) and release (k2) variables; therefore, DV=K1/k2. The assumption was made that the tissue DV of [11C](+)McN-5652 consisted of three components: specific binding, non-specific binding, and free ligand, whereas the DV of [11C](-)McN-5652 consisted of only non-specific binding and free ligand.
We used logarithmic transforms of DVs corrected for non-specific binding to achieve a normal distribution in the control and MDMA groups, and to enable analysis of variance. Logarithmic transformation across all regions in participants in the control group resulted in a pooled coefficient of variation of 22%.
Statistics
We tested the main effect of MDMA use on compartmental variables derived from 12 brain regions by general linear model-based MANOVA, with age and sex as covariates. Differences of individual regional variables were tested by one-way ANOVA. We investigated the relation between radioligand binding, extent of previous MDMA use, and duration of abstinence from MDMA with Pearson's correlations p<0·05 was taken to be significant with a two-tailed test. We analysed all data with SPSS version 7.5.Results
The two groups were similar for age, sex distribution, and level of education (table 1).| Controls (n=15) | MDMA (n=14) | |
| Mean (SD) age (years) | 28·3 (11·7) | 26·6 (10·5) |
| Mean (SD) education (years) | 16 (4) | 15 (2) |
| Men/women | 9/6 | 9/5 |
| Table 1: Characteristics of participants | ||
In the MDMA group, participants had generally used MDMA on more than 200 occasions and over 4-5 years. Most of the participants had not used MDMA for months, and some had not used MDMA for several years (table 2).
| Number of times used | 228 (70-400) |
| Duration of use (years) | 4·60 (1·5-10·0) |
| Usual dose (mg)* | 386 (150-1250) |
| Frequency of use/month | 6 (1-16) |
| Time since last dose (weeks) | 19 (3-147) |
| *Based on number of tablets or capsules ingested per day (100 mg=one tablet or capsule). | |
| Table 2: Mean (range) values for MDMA use | |
The injected doses of radioligands did not differ significantly in the two groups (table 3). We plotted representative time-activity curves with model fits for [11C](+)McN-5652 and [11C](-)McN-5652 for the two groups (figure 1). Although control and MDMA participants had similar transport of [11C]McN-5652 from blood to brain (K1) for all brain regions assessed, MDMA users had significant global decreases in DVs for specific binding of [11C]McN-5652 (p=0·011), which suggests that they had a lower density of brain 5-HT transporter sites than participants in the control group (figure 2). The covariance effects of age and sex were not significant (p=0·95 and p=0·16, respectively). Decreases of regional brain DVs in human MDMA users (figure 3) are consistent with PET findings in MDMA-treated baboons.23
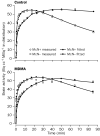 |
Shown are measured and model fitted data for both [11C]McN-5652 enantiomers. |
| Injected dose (µg) | Injected dose (MBq) | Specific activity (MBq/µmole) | ||||
| (+)[11C]McN | (-)[11C]McN | (+)[11C]McN | (-)[11C]McN | (+)[11C]McN | (-)[11C]McN | |
| Controls (n=15) | 3·56 (0·33) | 3·68 (0·50) | 688·2 (14·8) | 692·0 (10·0) | 80290 (9953) | 83324 (9509) |
| MDMA (n=14) | 3·51 (0·25) | 3·83 (0·44) | 677·1 (11·1) | 680·8 (11·1) | 75406 (5994) | 76183 (11137) |
| Table 3: Mean (SE) values of injected doses | ||||||
Decreases in 5-HT transporter binding positively correlated with extent of previous MDMA use, (r=-0·50, p=0·005, figure 4), consistent with data in animals of exposure-related loss of 5-HT terminals after MDMA administration.1,2,5-8 There was no correlation between the duration of abstinence from MDMA and the extent of specific [11C]McN-5652 binding (r=-0·09, p=0·75). All participants in the MDMA group were negative for recent drug use.
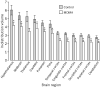 |
Data are mean (SE). *p<0·05. |
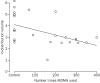 |
Controls are shown as no exposure. |
Discussion
Recreational MDMA use can lead to global, dose-related decreases in the brain 5-HT transporter, a structural element of brain 5-HT neurons. Taken in conjunction with results of previous studies showing selective decreases in concentrations of cerebrospinal fluid 5-hydroxindoleacetic acid in MDMA users15,16 and similar findings in MDMA-treated animals with documented neurotoxic lesions,1-9,17,24 these data suggest that human MDMA users are susceptible to MDMA-induced brain 5-HT neural injury.All participants in the MDMA group in our study reported that they had abstained from use of MDMA or other psychoactive drugs for at least 3 weeks before the study, which suggests that the decreases we saw in brain [11C]McN-5652-labelled 5-HT-transporter sites were not due to pharmacological effects of MDMA or other drugs. Our results do not, however, rule out the possibility that decreased 5-HT-transporter binding sites are secondary to pre-existing differences in 5-HT function in MDMA users compared with controls, but since none of the MDMA users had a neuropsychiatric disorder in which 5-HT has been implicated, this possibility is unlikely. Finally, although most of the MDMA users had experimented with other recreational drugs, none was a known 5-HT neurotoxin in human beings, and was not likely to account for changes in 5-HT binding.
Our data do not allow conclusions about reversibility or permanence of MDMA-induced changes in brain 5-HT transporter. Although we found no correlation between the duration of abstinence and the extent of decrease in [11C]McN-5652 binding, MDMA-induced changes may be reversible. Sample sizes and various other factors could have contributed to the apparent absence of recovery. More MDMA users with varied durations of abstinence and drug exposure histories must be studied to show whether 5-HT-terminal structure and function return to normal over time. Studies in non-human primates show that MDMA-induced changes in 5-HT terminal markers persist for longer than 1 year after doses of MDMA similar to those used by some human MDMA users.10,11,14
Our finding that a selective 5-HT transporter ligand can be used to show the pathophysiological state of 5-HT neurons in living human brains might have broad implications for many neuropsychiatric illnesses in which brain 5-HT neurons have been implicated, including depression, anxiety, and cognitive dysfunction.33,34 Quantitative PET imaging studies with [11C]McN-5652 should help to define the role of alterations in the 5-HT transporter in the basic pathophysiology of 5-HT-linked neuropsychiatric disorders and their response to treatment with selective 5-HT reuptake inhibitors.
A potential drawback of our study is that we relied upon participants' reports of drug use and duration of abstinence. This is an inherent problem in all studies of illicit drug use. Our participants are unlikely, however, to have altered their drug-use history because they contacted us and identified themselves as MDMA users. Drug tests indicated that no participant used marijuana in the 2-3 weeks before the study, despite several participants being regular marijuana users. Participants were told that they would undergo drug testing at the time of admission to the study, and that they would not receive payment if they had positive results for psychoactive drugs. Knowledge of drug testing upon arrival was probably a good deterrent from drug use, and because participants underwent PET scans 48 h after drug testing, we can be certain that participants abstained from amphetamines for 4 days before PET imaging. In future studies, hair-sample analysis would be a more useful way to ascertain long periods of abstinence from MDMA.
Sex differences in the effects of MDMA exposure on [11C]McN-5652 binding should be studied further. Our analysis showed no differences between men and women in the two groups. Nevertheless, the sample was small, and differences might be found if more women were studied. No sex differences in susceptibility to MDMA neurotoxic effects have been found in animals, but additional studies with participants matched for age and sex are necessary.
In summary, our data suggest that people who use MDMA as a recreational drug are unwittingly putting themselves at risk of developing brain 5-HT neural injury. In addition, systematic studies of MDMA-exposed individuals with highly selective brain 5-HT transporter deficits may give important insights into the functional role of brain 5-HT in human behaviour. Potential functional consequences of MDMA-induced brain 5-HT neurotoxic lesions are not yet clear, but may include depression, anxiety, memory disturbance, and other neuropsychiatric disorders in which brain 5-HT has been implicated.33,34
Contributors
U D McCann provided psychiatric oversight and was the principal author of the paper. Z Szabo did the PET studies with [11C]McN-5652, modelled the ligand, analysed PET data, and contributed substantially to the preparation of the paper. U Scheffel assisted with the execution of the PET studies, played a key role in the analyses of high-performance liquid chromatography, and contributed to the preparation of the paper. R F Dannals directed the execution of the PET studies and assisted in the preparation of the paper. G A Ricaurte was the principal investigator and was responsible for neurological assessment and general oversight of the study, and contributed substantially to the preparation of the paper.Acknowledgments
This work was supported by Public Health Service grants DA10217, DA05938, DA06275, (GAR), AG14400 (ZS) MOIRR02719(GCRC) and the National Institute of Mental Health IRP (UDM).References
1 Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther 1987; 240: 1-7.2 Commins DL, Vosmer G, Virus R, Woolverton W, Schuster C, Seiden L. Biochemical and histological evidence that methylenedioxymethamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther 1987; 241: 338-45.
3 Battaglia G, Yeh SY, O'Hearn E, Molliver ME, Kuhar MJ, DeSouza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labelled serotonin uptake sites. J Pharmacol Exp Ther 1987; 242: 911-16.
4 O'Hearn EG, Battaglia G, DeSouza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci 1988; 8: 2788-803.
5 Slikker W, Ali SF, Scallet C, Frith CH, Newport GD, Bailey JR. Neurochemical and neurohistological alterations in the rat and monkey produced by orally administered methylenedioxymethamphetamine (MDMA). Toxicol Appl Pharmacol 1988; 94: 448-57.
6 Ricaurte GA, Forno LS, Wilson MA, et al. (±)3,4-methylenedioxymethamphetamine (MDMA) selectively damages central serotonergic neurons in non-human primates. JAMA 1988; 260: 51-55.
7 Ricaurte GA, DeLanney LE, Irwin I, Langston JW. Toxic effects of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration. Brain Res 1988; 446: 165-68.
8 Insel TR, Battaglia G, Johannessen JN, Marra S, De Souza EB. 3,4-Methylenedioxymethamphetamine ("ecstasy") selectively destroys brain serotonin terminals in rhesus monkeys. J Pharmacol Exp Ther 1989; 249: 713-20.
9 Molliver ME, Berger UV, Mamounas LA, Molliver DC, O'Hearn EG, Wilson MA. Neurotoxicity of MDMA and related compounds: anatomic studies. Ann NY Acad Sci 1990; 600: 640-64.
10 Ricaurte GA, Martello AL, Katz JL, Martello MB. Lasting effects of (±)3,4-methylenedioxymethamphetamine on central serotonergic neurons in non-human primates: neurochemical observations. J Pharmacol Exp Ther 1992; 261: 616-22.
11 Fischer CA, Hatzidimitriou G, Katz JL, Ricaurte GA. Reorganization of ascending serotonin axon projections in animals previously exposed to the recreational drug 3,4-methylenedioxymethamphetamine. J Neurosci 1995; 15: 5476-85.
12 Ricaurte GA. Studies of MDMA-induced neurotoxicity in nonhuman primates: a basis for evaluating long-term effects in humans. In: Asghar K, DeSouza E, eds. Pharmacology and toxicology of amphetamines and related designer drugs. NIDA Res Monogr 1989; 94: 306-22.
13 Eisner B. Ecstasy: the MDMA Story. Berkeley: Ronin Publishing, 1989.
14 Chappell W, Mordenti J. Extrapolation of toxicological and pharmacological data from animals to humans. In: Tests B, ed. Advances in drug research. San Diego: Academic Press, 1991: 1-116.
15 Ricaurte GA, Finnegan KF, Irwin I, Langston JW. Aminergic metabolites in cerebrospinal fluid of humans previously exposed to MDMA: preliminary observations. Ann NY Acad Sci 1990; 600: 699-708.
16 McCann UD, Ridenour A, Shaham Y, Ricaurte GA. Serotonin neurotoxicity after MDMA: a controlled study in humans. Neuropsychopharmacology 1994; 10: 129-38.
17 Ricaurte GA, DeLanney LE, Wiener SG, Irwin I, Langston JW. 5-Hydroxyindoleacetic acid in cerebrospinal fluid reflects serotonergic damage induced by 3,4-methylenedioxymethamphetamine in CNS of non-human primates. Brain Res 1989; 474: 359-63.
18 Brewerton TD, Berrettini WH, Nurnberger JI, Linnoila M. Analysis of seasonal fluctuations of CSF monoamine metabolites and neuropeptides in normal controls: findings with 5-HIAA and HVA. Psychiatr Res 1989; 23: 257-65.
19 Dannals RF, Scheffel U, Suehiro M, Ricaurte G. Radioligand development for studying serotonin uptake sites with positron emission tomography. Med Chem Res 1994; 5: 228-44.
20 Suehiro M, Scheffel U, Dannals RF, Ricaurte GA, Ravert HT, Wagner HN. A PET radiotracer for studying serotonin uptake sites: [11C]McN-5652-Z. J Nucl Med 1993; 34: 120-27.
21 Szabo Z, Sceffel U, Suehiro M, et al. Positron emission tomography of serotonin transporter sites in baboon brain with [11C]McN5652. J Cereb Blood Flow Metab 1995; 15: 798-805.
22 Szabo Z, Kao P, Scheffel U, et al. Positron emission tomography of serotonin transporter sites in human brain with [11C]McN5652. Synapse 1995; 20: 37-43.
23 Scheffel S, Szabo Z, Matthews W, Dannals R, Ravert H, Ricaurte G. Fenfluramine-induced loss of serotonin transporters in baboon brain visualized with PET. Synapse 1996; 24: 395-98.
24 Scheffel U, Szabo Z, Mathews WB, et al. In vivo detection of short- and long-term MDMA neurotoxicity: a positron emission tomography study in the living baboon brain. Synapse 1998; 29: 183-92.
25 Scheffel U, Szabo Z, Mathews W, et al. Validation of compartmental and noncompartmental kinetic models for [11C]McN5652 binding in baboons. Soc Neurosci Abstracts 1997; 23: 558.
26 Suehiro M, Ravert HT, Dannals RF, et al. Synthesis of a radiotracer for studying 5-HT uptake sites with positron emission tomography: [11C]McN-5652-Z. J Lab Comp Radiopharm 1992; 31: 841-48.
27 Bergström M, Eriksson L, Bohm C, Blomqvist G, Litton J. Correction for scattered radiation in a ring detector positron camera by integral transformation of the projections. J Comput Assist Tomogr 1983; 7: 42-50.
28 Frey KA, Koeppe RA, Kilbourne MR, et al. Presynaptic monoaminergic vesicles in Parkinson's disease and normal aging. Ann Neurol 1996; 40: 873-84.
29 Koeppe RA, Frey KA, Van der Borght TM, et al. Kinetic evaluation of [11C]dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab 1996; 16: 1288-99.
30 Muzik O, Chugani D, Chakraborty O, et al. Analysis of C-11 alpha-methyl-tryptophan kinetics for estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab 1997; 17: 659-69.
31 Marquardt DW. An algorithm for least-squares estimation of nonlinear parameters. J Soc Industr Appl Math 1963; 11: 431-41.
32 Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical recipes. In: The art of scientific computing. Cambridge, USA: University Press, 1986.
33 Coccaro EF, Murphy DL. Serotonin in Major Psychiatric Disorders. Washington, DC: American Psychiatric Press, 1990.
34 Sandler M, Coppen A, Harnett S. 5-Hydroxytryptamine in psychiatry: a spectrum of ideas. Oxford: Oxford University Press, 1991.