Novel Drug Briefs
New Entries in the Rapidly Expanding Market (AL-LAD, LSZ, 5-IT, 5-MAPB, TCB-2, Pyrazolam, and Flubromazepam)
December 2013
Originally published in Erowid Extracts #25
Citation: Abrad. "Novel Drug Briefs: New Entries in the Rapidly Expanding Market (AL-LAD, LSZ, 5-IT, 5-MAPB, TCB-2, Pyrazolam, and Flubromazepam)". Erowid Extracts. December 2013;25:12-16. Online edition: Erowid.org/chemicals/chemicals_article1.shtml
See also: the second installment of Novel Drug Briefs and Recreational Roulette
As part of the Erowid crew, I monitor various public and private forums as well as news articles, vendor websites and incoming experience reports to try and stay abreast of current trends in the use of psychoactives. I pay particular attention to rare or novel substances and those with limited available experiential data in humans.
Some of the newer compounds to emerge through online research chemical suppliers have previously never been seen outside of the laboratory where they had been used in receptor binding assays, while others are drugs that have been in clinical use, or are derivatives of medicinal drugs.
New research chemicals are often described glowingly in the first few public experience reports. This can create an anticipatory buzz online and make initial assessments unbalanced. Erowid has dealt with this issue for nearly 15 years. It's best to wait to form impressions until enough voices have joined the discussion to temper initial enthusiasm or vendor bias.
I've chosen two ergoloids (AL-LAD and LSZ), an indole derivative (5-IT), two phenethylamine relatives (2C-EF and TCB-2), a new entactogen (5-MAPB), and two novel benzodiazepines (flubromazepam and pyrazolam). These eight substances are representative of the broad range of novel compounds recently made available in the current marketplace.
AL-LAD is an LSD analog that was first synthesized in the mid 1970s by Niwaguchi and Nakahara, and then studied in the 1980s by the Nichols lab at Purdue, where it was compared with LSD using rat discrimination research. AL-LAD was included in Alexander and Ann Shulgin's 1997 book, TiHKAL. It became available through an underground source in early May 2013, and was being sold by several research chemical vendors in the UK within weeks.
Initial descriptions of its effects by those who purchased the early batches were very positive. True to its description in TiHKAL, AL-LAD seems to be slightly less potent than LSD, with reports pointing to a shorter duration and greater euphoria than LSD. As of November 2013, it was being sold by UK vendors at £69 ($111 USD) for ten 150-µg blotter tabs.
The following report, written by Sepher [RIP - July 2, 2013], was posted on Bluelight and a UK-based forum in late May 2013 and submitted to Erowid shortly after.
T+0:00: ~450 µg, or as close as I can be cutting blotter. Applied upper lip, see if buccal admin comes up any faster. I proved it's orally active last week with a 300 µg oral dose.
T+1:00: First signs of a visual disturbance, patches of colour coming at the very edges of vision. Buzz mainly at the temples coming in. Very vague at this point.
T+3:00: Developed really fast, from T+2:30 on, seemed to suddenly come up in a great big rush. Massive rush of colour, patterns exploding all over visual field, stacking up over each other. Lots of vibration to everything. Long tracers swirling my hands about. Bedroom door looks jellified, wobbling and melting in its doorframe, rippling away. Really good, detailed CEVs, beautifully intricate with dynamic fractal patterns. Brightly lit, colours include lots of pale pinks and yellows, kinda neon. Thrashing around a bit, breathing hard helps bring massive rushes of euphoria with waves of warmth coming up from the body and through the head. The images seem to surge with the motion of my head.
T+4:00: Weird thinking very much in evidence. Caught myself a couple of times going down long meanders that don't make sense. The chains of thoughts from one thing to another don't link up. I don't understand the thinking going on. Some of it is irrational, with hints of the madhouse I sometimes get with LSD, that kind of "have I gone mad" questioning where I can't make sense of things. Little time-out moments where I have to just stop and focus, stop all thought, and try and pull back to something I have a proper handle on. Not that easy at times. I have to exert some real effort to wrest control back. Very meditative, emotionally connected. Music sounds incredible. Massive distortions with huge flanging effects, pitch-shifting, and tempo all over the place at times. Moments where it was just waves of sound washing over me. The shape of each one evident in minute detail [...].
T+6:00: Past peak now. Still very visual and colourful. CEVs have dropped off in intensity, not so engaging. Still there but can't melt into them anymore. They're outside of me again, eye-candy only. Still can barely see to type, but easier. Very euphoric still, but more relaxed with it, calmer, not rushy anymore. Still a nice buzz to it.
T+8:00: Still going, not faded completely yet. Colour patterns still in evidence, all OEV now, with CEVs almost gone [...] Think will sleep despite there still being some of the buzz going. It's not stimulation as such.
Other reports followed, many of them similarly positive. AL-LAD has only been available through a small number of vendors, perhaps due to its relatively difficult synthesis process. However, that it is available at all is an interesting development since synthetic recreational ergoloids other than LSD have never before been available on such a scale.
Another ergoloid, LSZ, also hit the market in mid-2013, and at the time of writing is available from many of the same sources as AL-LAD. It was developed by David Nichols's lab at Purdue in the early 2000s. There is little experiential data on it.
One experience report by Brother Wolf published on Erowid in July 2013, indicates that it has a longer duration than AL-LAD with a slower onset and a roughly similar potency.
T+0:00: A half blotter, 75 µg, tucked in my gums (buccal).
T+1:45: Gradual increase in and building of effects noted. Still extremely subtle but I may have caught some imprinting when glancing at and away from a monochrome fractal poster on my wall. There seems to be a little more red, pink, and orange in my visible spectrum than might usually be present. I continue to kick back. Still very comfortable, mentally and physically, a very nice, relaxing way to spend a gorgeous summer Saturday.
T+2:15: Visuals are picking up a little and my mind is less clear now, foggier, heavier. Hand-eye coordination has also been affected. Typing is slightly trickier than it was 15 minutes ago. The appearance of tracers and other common visual activity has increased.
T+3:45: Wow, fully immersed now. This shit is a slow-burner, and it's reminding me a little bit of a DOx type experience.
T+5:15: Still pretty busy here. I think the effects peaked about half an hour ago, I feel as though I might be on the downslope now. Not certain or anything, if this is anything like the original L it's bound to ease off in waves, much like it started...
T+10:15: Definitely easing off now, I expect to be completely baseline in an hour. I expect easy sleep before midnight.
A report by Borax, submitted to Erowid in July 2013, notes a total duration of about 8 hours with a 3-hour onset.
It took approximately 45-60 minutes until the first alert, 3 hours to peak and a total duration of perhaps 8 hours plus afterglow. With a higher dose it may reveal itself to last longer and come up a little faster.
5-IT is a chemical related to AMT that was first described by Albert Hofmann and Franz Troxler in 1963. It became available from chemical suppliers in the United Kingdom, Sweden, and Spain in early 2012. Initial reports suggested its effects were similar to AMT but "dirtier" and "less visual", with a duration of about 10 hours. Many positive reports were written in the following months describing doses up to 400 mg being taken without incident. Users reported taking it in combination with compounds such as 6-APB and 5-MeO-MiPT, among others.
Displaying empathogenic effects similar to MDA and 5-APB, 5-IT quickly gained popularity despite concerns about psychotic reactions and cardiovascular problems. These concerns have turned out to be justified.
In July 2012, a popular chemical supply company posted a notice on their website saying, "We have decided not to sell this chemical anymore, we heard about to[o] much abuse! We are sorry, but we cannot allow our selfs [sic] to ruin our image because of one chemical! We hope for your understanding!"
Later that month a news report surfaced linking 5-IT to 14 deaths in Sweden, with it being found to be the sole intoxicating agent in three of the cases. 1 A short time later, most chemical suppliers began discontinuing sales of 5-IT.
In late August 2012, a member of a UK-centric forum reported that a member had died after taking 5-IT. BMJ then published a letter reporting that 5-IT was identified in the postmortem blood of two adults. 2
An additional hospitalisation was reported in October, though this seems to have been a case of 5-IT overdose following a stimulant binge.
In late 2012, the Belgian Early Warning System website published a warning hinting at one possible explanation for the Swedish deaths, "It seems that recently, a batch of 5-IT has been mislabeled, and was sold on the internet as 5-APB or 6-APB. Considering the difference in dosage, a lot of users in Sweden thought they were consuming APB, and, consequently, overdosed, with 14 deaths reported in 2012."
Because of concerns over safety and the resulting reduced availability, 5-IT fell out of favor with vendors and users and was banned as a Temporary Class Drug in the UK in 2013.
In November 2013, 2C-EF caused excitement on forums due to some glowing experiences reported a few years ago coupled with the expectation that it would soon become available from at least one online chemical supplier.
Though lacking its own PiHKAL entry, Shulgin does mention it under DOEF as the 2-carbon analog, noting that "it would be every bit as much a treasure and ally as is 2C-B or 2C-I".
Two very enthusiastic reports posted on Bluelight in 2010, along with a mention in PiHKAL, generated an air of anticipation on forums about its possible availability.
In September 2010, Fastandbulbous posted a description to Bluelight, titled "Another wonderful drug experience", describing the effect of 10 mg 2C-EF combined with 20 mg methoxetamine as "unexpectedly strong".
T+0:00: Swallowed 10 mg in some orange juice.
T+0:15: First alerts noticed (on top of 20 mg of methoxetamine)
T+0:30: Visual patterning very noticeable. I thought 2C-D was better than 2C-B, but this is much, much more in every department.
If I had to make a conclusion, it would be that this is the best 2C-x compound I'd ever taken, a real quality psychedelic up there with mescaline, DOM, and LSD. In fact, given the choice, I'd take 2C-EF over those three on most occasions. Thank fuck that I decided to dose on the low side. I'd hate to think what sort of experience would erupt from taking 20 mg. Either overwhelmingly positive or a trip to hell.
2C-EF left 2C-B/2C-E standing in the dust IMO. Shame it's illegal in the UK as this would be my first choice for a night of dancing/spiritual revelation. My cynical side is still looking for a negative aspect to the experience, but I still haven't found one!
A few days later, an experimenter named B9 submitted his own report, "It's a wonderful night", to Bluelight.
10 mgs dosed in juice for me and my friend, Kevin. The setting was my house during a weekday afternoon with my kids and dogs roaming about asking questions and barking, respectively.
There's definite activity at 20 minutes. At 30 minutes I consumed another 5 mg. I feel a little lightheaded and a good humour has come over me. During the next hour the effects continue to increase with visual patterning on the walls and inside of my eyelids. The conversation as I recall it was mainly centered around how good the music we were listening to sounded. This stuff seems to enhance nearly every sense, music sounds incredibly good, the grapefruit I ate was a thing of wonder as my teeth sank slowly through the juicy flesh. I felt like it was as good as the first time I ever ate a citrus fruit.
For such a strong experience the drug produced no noticeable physical side effects. At no point was my pulse elevated. There was no increase in body temperature, no sweating or shivering, no feeling of artificially induced stimulation or artificially induced euphoria, unlike say MDMA. The whole good mood thing was just that. It felt as near natural as it possibly could.
First appearing in December 2012, 5-MAPB quickly gained repute as being very close in effects to MDMA, with users noting that they wouldn't be able to tell the difference between MDMA and 5-MAPB in a blind test.
It seems to be strongly active at ~100 mg, making it roughly the same potency as MDMA. It is also reportedly more stimulating than either 6-APB or 5-APB.
Sepher commented on Bluelight:
It's the closest RC I've yet found [to MDMA] and it produced very good results in a club. Effects were broadly similar but it's still not quite there. Not quite as full on as far as energy, euphoria and empathy are concerned. You'd probably feel there was something missing if you took it expecting an exact substitute and be disappointed by it. Take it for what it is though. I think it's a very worthwhile substance.
First synthesized by Thomas McClean in the Nichols lab at Purdue University in 2006, TCB-2 is a derivative of 2C-B. TCB-2 appears to be extremely potent, as much or more so as DOI and with a total duration of longer than 24 hours. At this time, there are few reports of recreational use, though this could change if more vendors choose to distribute it.
In "TCB-2 Overview", published on Erowid in September 2012, BilZ0r describes a series of increasing doses with TCB-2 hydrobromide salt:
T+0:00: 250 µg ingested orally.
T+1:55: Possible alert. Unsure. Nothing else to report. Slept well.
The second day:
T+0:00: 750 µg ingested orally.
T+1:15: Clear alert. I sense the classic body signals I feel when I take hallucinogens, an uncom¬fortable sensation in my neck.
T+2:15: There has been no progression. No visual or thought disturbances.
T+3:15: Body signals dissipate.
The third day:
T+0:00: 3 mg insufflated. As expected, this is quite painful. The pain continues in my sinus for 15 minutes while I resist sneezing or blowing my nose.
T+2:04: First alert.
T+3:04: Nothing greater to report. Wondering if the previous day's effect was imagined. I settle in to
watch a movie.
T+3:34: I smoke a small amount of marijuana (quarter of a bowl). Feel very, very stoned. Having difficulty expressing myself. Realize I am having subtle visual hallucinations when looking at faces. Oddly, no CEVs.
T+5:04: Still having subtle hallucinations and difficulty expressing my thoughts when speaking. This is definitely a mild psychedelic experience.
T+5:34: Take 3.5 mg of zopiclone to sleep. I wake the next day feeling fine.
One week later:
T+0:00: Ingest 5 mg orally with S (a 110 lb female). Begin to make a stew, assuming it would take several hours to kick in as it did previously.
T+0:13: First alert. This is not going to take hours to kick in.
T+0:28: The classic body sensations continue to mount for half an hour. No visuals to report, however I am having significant difficulties in expressing myself succinctly. S reports that she felt it kick in within 15 minutes, but didn't want to say anything after I had claimed, "it will take several hours to have an effect". I timed my dose with the with the assumption that it would be several hours before I felt effects, however, I am already significantly disorientated as I make the bean stew. Thankfully I just get it done before I start to experience coordination difficulties.
T+0:33: Open eye visuals are beginning to start (interestingly, no CEVs). Not currently what I would call classic psychedelic effects, but effects like the TV screen seeming further from me than I know it is, or other size and scale distortions.
T+0:43: Full psychedelic state. ++/+++. Large degree of visual hallucinations, fractals patterns, apparent movement of details, etc.
T+12:45: The ++/+++ state continued for the last 12 hours. I was without anxiety, depression or nausea. S reports similarly. She had a slightly higher degree of visual hallucinations. Insignificant side effects are very shaky hands. S is very stimulated and declares she will not be able to sleep. I am unsure if I will be able to. We both ingest approximately 10 mg of zopiclone to sleep.
T+23:00: The next day, there are still subtle visual effects, like visual snow. We are both tired and groggy. I develop a rather bad headache. S does not. I attribute the headache to dehydration due to general partying over the preceding days. The next day the headache is gone, and I feel fine.
Conclusions: TCB-2 does not appear to be the ultra potent hallucinogen some of its pharmacological properties suggest it should be. It appears to be somewhat less potent than DOI.
Documenting and determining dosage ranges in humans is difficult when only a small number of experience reports are available. This is one of the major issues faced when collecting data on such novel compounds. While BilZ0r speculates that TCB-2 is less potent than DOI (which is active at around a milligram), an author on Bluelight describes TCB-2 as fully active at 250 µg (0.25 mg). Potency extrapolated from drug substitution studies in rats suggests its dosage is on par with LSD or Bromo-Dragonfly.
Developed in the 1970s as an anxiolytic, pyrazolam became available through online chemical suppliers in July 2012. Initial excitement over the potential similarity to the highly sought after benzodiazepines triazolom and alprazolam quickly turned to disappointment as reports posted on internet forums described it as weak, with little euphoric or recreational potential. As described by ScottishDNB on Bluelight in August 2012:
Pyrazolam does not seem to be very euphoric; the come-up is pretty drawn out.
Having 3 mg made me pretty intoxicated but if you have a tolerance they don't offer good value.
After trying pyrazolam, I would say it is a lot less euphoric than etizolam and diazepam although it shares the amnesia/blackout/clumsy traits and length of diazepam.
Flubromazepam is a novel benzodiazepine and analog of bromazepam that became available in late 2012. It has been compared to diazepam, though it appears to be more potent.
Initial reports posted to discussion forums were positive and drew comparisons to clonazepam, diazepam, alprazolam and etizolam. In late 2012, Mela wrote on Bluelight:
8 mg sublingual. 60 minutes later, initial sedative effect developed which evolved into mainly warm chilled anxiolysis by the 3rd hour. A few things happened later which, shall we say, meant it was difficult to distinguish its specific effects. Looks like a winner in the k-pin [klonopin] ballpark (given proposed 30 hour half-life). [...]
The onset effects are more in the diazepam ballpark (notably sedating). I tried 4 mg a few days later, and still found it sedating for an hour or so at this lower dose. For instance, I don't find clonazepam (1 mg), lorazepam (2 mg), alprazolam (1 mg), or even etizolam (at 1 mg) as sedating as 4mg of flubromazepam.
This compound seems likely to see increased use due to it recently becoming more widely available.
As part of the Erowid crew, I monitor various public and private forums as well as news articles, vendor websites and incoming experience reports to try and stay abreast of current trends in the use of psychoactives. I pay particular attention to rare or novel substances and those with limited available experiential data in humans.
Some of the newer compounds to emerge through online research chemical suppliers have previously never been seen outside of the laboratory where they had been used in receptor binding assays, while others are drugs that have been in clinical use, or are derivatives of medicinal drugs.
New research chemicals are often described glowingly in the first few public experience reports. This can create an anticipatory buzz online and make initial assessments unbalanced. Erowid has dealt with this issue for nearly 15 years. It's best to wait to form impressions until enough voices have joined the discussion to temper initial enthusiasm or vendor bias.
I've chosen two ergoloids (AL-LAD and LSZ), an indole derivative (5-IT), two phenethylamine relatives (2C-EF and TCB-2), a new entactogen (5-MAPB), and two novel benzodiazepines (flubromazepam and pyrazolam). These eight substances are representative of the broad range of novel compounds recently made available in the current marketplace.
AL-LAD
6-allyl-6-nor-lysergic acid diethylamide
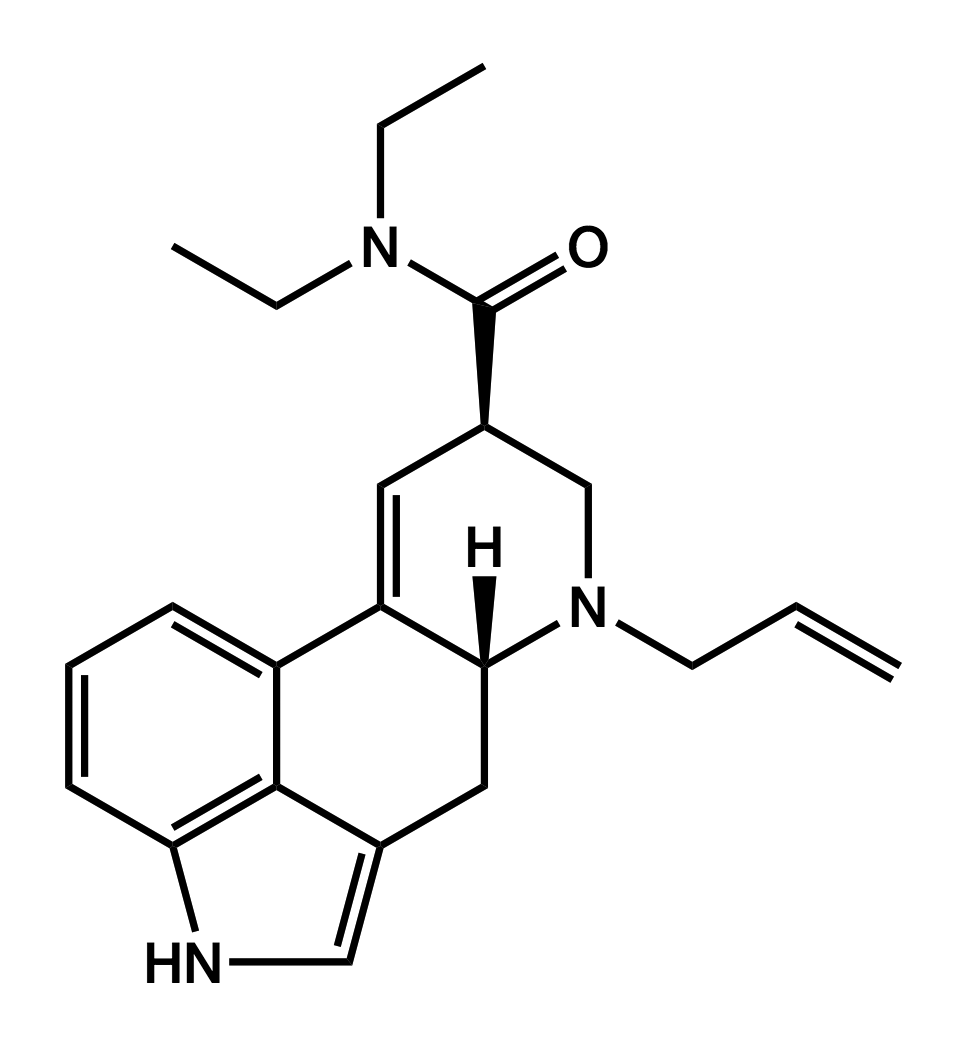
|
Initial descriptions of its effects by those who purchased the early batches were very positive. True to its description in TiHKAL, AL-LAD seems to be slightly less potent than LSD, with reports pointing to a shorter duration and greater euphoria than LSD. As of November 2013, it was being sold by UK vendors at £69 ($111 USD) for ten 150-µg blotter tabs.
The following report, written by Sepher [RIP - July 2, 2013], was posted on Bluelight and a UK-based forum in late May 2013 and submitted to Erowid shortly after.
T+0:00: ~450 µg, or as close as I can be cutting blotter. Applied upper lip, see if buccal admin comes up any faster. I proved it's orally active last week with a 300 µg oral dose.
T+1:00: First signs of a visual disturbance, patches of colour coming at the very edges of vision. Buzz mainly at the temples coming in. Very vague at this point.
T+3:00: Developed really fast, from T+2:30 on, seemed to suddenly come up in a great big rush. Massive rush of colour, patterns exploding all over visual field, stacking up over each other. Lots of vibration to everything. Long tracers swirling my hands about. Bedroom door looks jellified, wobbling and melting in its doorframe, rippling away. Really good, detailed CEVs, beautifully intricate with dynamic fractal patterns. Brightly lit, colours include lots of pale pinks and yellows, kinda neon. Thrashing around a bit, breathing hard helps bring massive rushes of euphoria with waves of warmth coming up from the body and through the head. The images seem to surge with the motion of my head.
T+4:00: Weird thinking very much in evidence. Caught myself a couple of times going down long meanders that don't make sense. The chains of thoughts from one thing to another don't link up. I don't understand the thinking going on. Some of it is irrational, with hints of the madhouse I sometimes get with LSD, that kind of "have I gone mad" questioning where I can't make sense of things. Little time-out moments where I have to just stop and focus, stop all thought, and try and pull back to something I have a proper handle on. Not that easy at times. I have to exert some real effort to wrest control back. Very meditative, emotionally connected. Music sounds incredible. Massive distortions with huge flanging effects, pitch-shifting, and tempo all over the place at times. Moments where it was just waves of sound washing over me. The shape of each one evident in minute detail [...].
T+6:00: Past peak now. Still very visual and colourful. CEVs have dropped off in intensity, not so engaging. Still there but can't melt into them anymore. They're outside of me again, eye-candy only. Still can barely see to type, but easier. Very euphoric still, but more relaxed with it, calmer, not rushy anymore. Still a nice buzz to it.
T+8:00: Still going, not faded completely yet. Colour patterns still in evidence, all OEV now, with CEVs almost gone [...] Think will sleep despite there still being some of the buzz going. It's not stimulation as such.
Other reports followed, many of them similarly positive. AL-LAD has only been available through a small number of vendors, perhaps due to its relatively difficult synthesis process. However, that it is available at all is an interesting development since synthetic recreational ergoloids other than LSD have never before been available on such a scale.
LSZ
lysergic acid (S,S)-2,4-dimethylazetidide
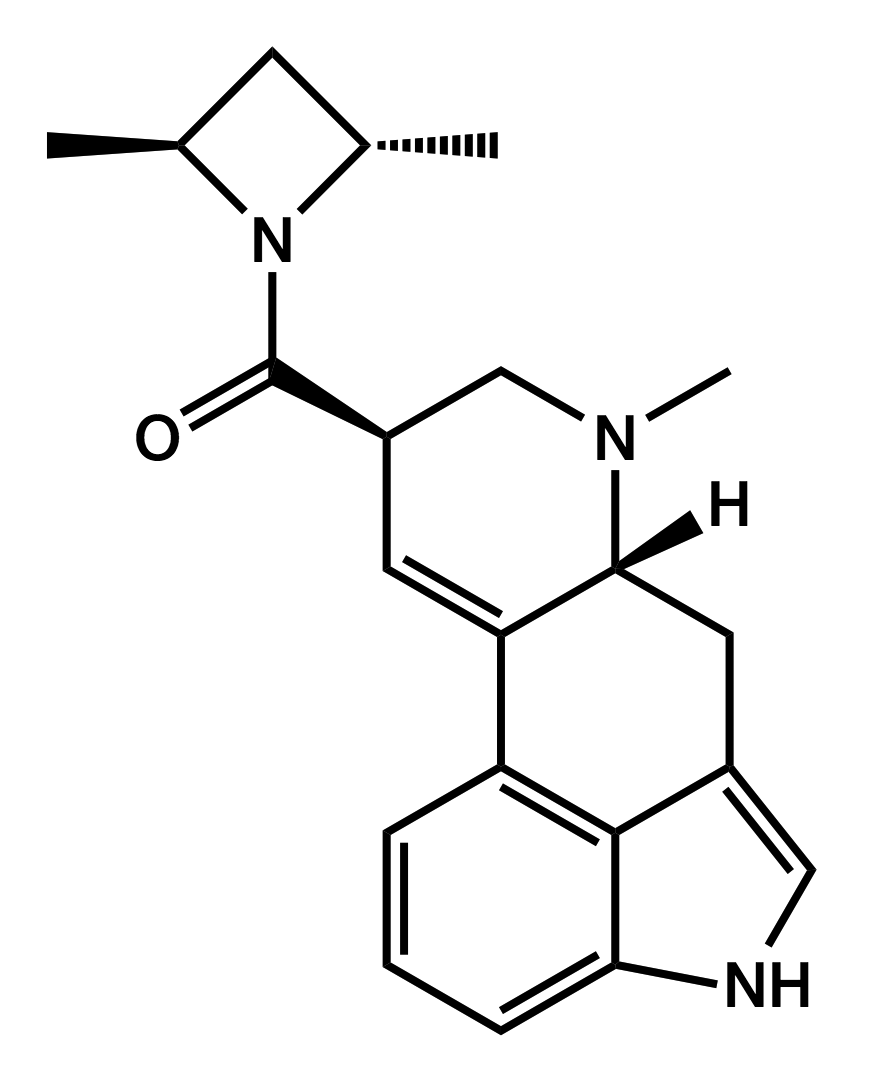
|
One experience report by Brother Wolf published on Erowid in July 2013, indicates that it has a longer duration than AL-LAD with a slower onset and a roughly similar potency.
T+0:00: A half blotter, 75 µg, tucked in my gums (buccal).
T+1:45: Gradual increase in and building of effects noted. Still extremely subtle but I may have caught some imprinting when glancing at and away from a monochrome fractal poster on my wall. There seems to be a little more red, pink, and orange in my visible spectrum than might usually be present. I continue to kick back. Still very comfortable, mentally and physically, a very nice, relaxing way to spend a gorgeous summer Saturday.
T+2:15: Visuals are picking up a little and my mind is less clear now, foggier, heavier. Hand-eye coordination has also been affected. Typing is slightly trickier than it was 15 minutes ago. The appearance of tracers and other common visual activity has increased.
T+3:45: Wow, fully immersed now. This shit is a slow-burner, and it's reminding me a little bit of a DOx type experience.
T+5:15: Still pretty busy here. I think the effects peaked about half an hour ago, I feel as though I might be on the downslope now. Not certain or anything, if this is anything like the original L it's bound to ease off in waves, much like it started...
T+10:15: Definitely easing off now, I expect to be completely baseline in an hour. I expect easy sleep before midnight.
A report by Borax, submitted to Erowid in July 2013, notes a total duration of about 8 hours with a 3-hour onset.
It took approximately 45-60 minutes until the first alert, 3 hours to peak and a total duration of perhaps 8 hours plus afterglow. With a higher dose it may reveal itself to last longer and come up a little faster.
5-IT
5-(2-Aminopropyl)indole
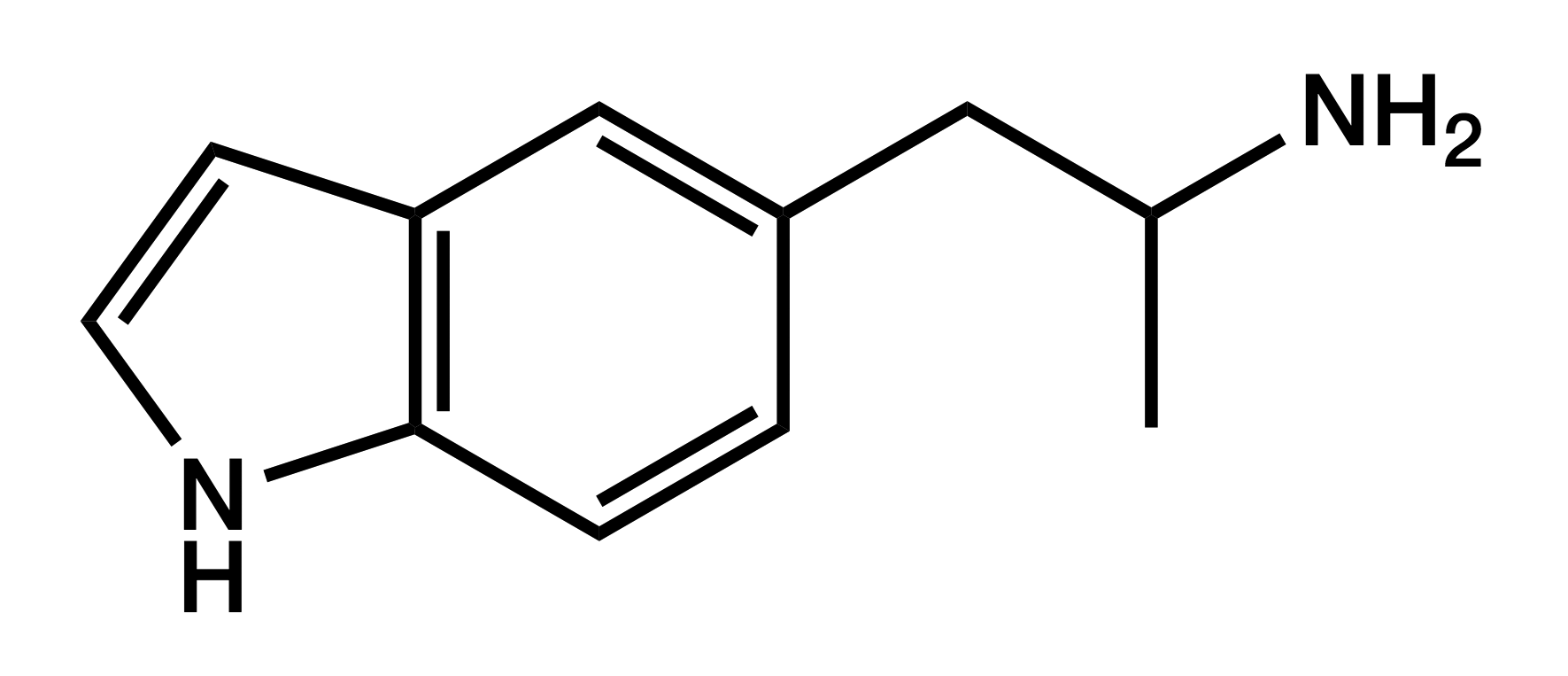
|
Displaying empathogenic effects similar to MDA and 5-APB, 5-IT quickly gained popularity despite concerns about psychotic reactions and cardiovascular problems. These concerns have turned out to be justified.
In July 2012, a popular chemical supply company posted a notice on their website saying, "We have decided not to sell this chemical anymore, we heard about to[o] much abuse! We are sorry, but we cannot allow our selfs [sic] to ruin our image because of one chemical! We hope for your understanding!"
Later that month a news report surfaced linking 5-IT to 14 deaths in Sweden, with it being found to be the sole intoxicating agent in three of the cases. 1 A short time later, most chemical suppliers began discontinuing sales of 5-IT.
In late August 2012, a member of a UK-centric forum reported that a member had died after taking 5-IT. BMJ then published a letter reporting that 5-IT was identified in the postmortem blood of two adults. 2
An additional hospitalisation was reported in October, though this seems to have been a case of 5-IT overdose following a stimulant binge.
In late 2012, the Belgian Early Warning System website published a warning hinting at one possible explanation for the Swedish deaths, "It seems that recently, a batch of 5-IT has been mislabeled, and was sold on the internet as 5-APB or 6-APB. Considering the difference in dosage, a lot of users in Sweden thought they were consuming APB, and, consequently, overdosed, with 14 deaths reported in 2012."
Because of concerns over safety and the resulting reduced availability, 5-IT fell out of favor with vendors and users and was banned as a Temporary Class Drug in the UK in 2013.
2C-EF
4-fluoroethyl-2,5-methoxyphenethylamine
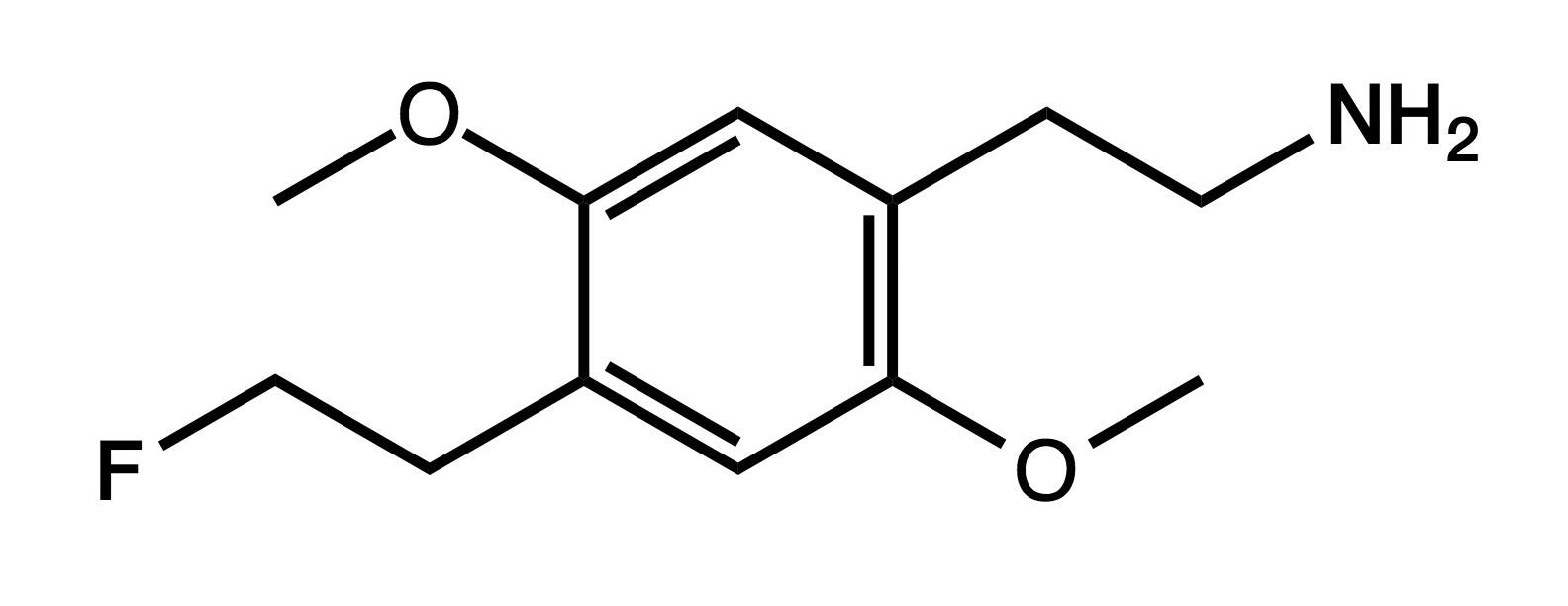
|
Though lacking its own PiHKAL entry, Shulgin does mention it under DOEF as the 2-carbon analog, noting that "it would be every bit as much a treasure and ally as is 2C-B or 2C-I".
Two very enthusiastic reports posted on Bluelight in 2010, along with a mention in PiHKAL, generated an air of anticipation on forums about its possible availability.
In September 2010, Fastandbulbous posted a description to Bluelight, titled "Another wonderful drug experience", describing the effect of 10 mg 2C-EF combined with 20 mg methoxetamine as "unexpectedly strong".
T+0:00: Swallowed 10 mg in some orange juice.
T+0:15: First alerts noticed (on top of 20 mg of methoxetamine)
T+0:30: Visual patterning very noticeable. I thought 2C-D was better than 2C-B, but this is much, much more in every department.
If I had to make a conclusion, it would be that this is the best 2C-x compound I'd ever taken, a real quality psychedelic up there with mescaline, DOM, and LSD. In fact, given the choice, I'd take 2C-EF over those three on most occasions. Thank fuck that I decided to dose on the low side. I'd hate to think what sort of experience would erupt from taking 20 mg. Either overwhelmingly positive or a trip to hell.
2C-EF left 2C-B/2C-E standing in the dust IMO. Shame it's illegal in the UK as this would be my first choice for a night of dancing/spiritual revelation. My cynical side is still looking for a negative aspect to the experience, but I still haven't found one!
A few days later, an experimenter named B9 submitted his own report, "It's a wonderful night", to Bluelight.
10 mgs dosed in juice for me and my friend, Kevin. The setting was my house during a weekday afternoon with my kids and dogs roaming about asking questions and barking, respectively.
There's definite activity at 20 minutes. At 30 minutes I consumed another 5 mg. I feel a little lightheaded and a good humour has come over me. During the next hour the effects continue to increase with visual patterning on the walls and inside of my eyelids. The conversation as I recall it was mainly centered around how good the music we were listening to sounded. This stuff seems to enhance nearly every sense, music sounds incredibly good, the grapefruit I ate was a thing of wonder as my teeth sank slowly through the juicy flesh. I felt like it was as good as the first time I ever ate a citrus fruit.
For such a strong experience the drug produced no noticeable physical side effects. At no point was my pulse elevated. There was no increase in body temperature, no sweating or shivering, no feeling of artificially induced stimulation or artificially induced euphoria, unlike say MDMA. The whole good mood thing was just that. It felt as near natural as it possibly could.
5-MAPB
1-(benzofuran-5-yl)-N-methylpropan-2-amine
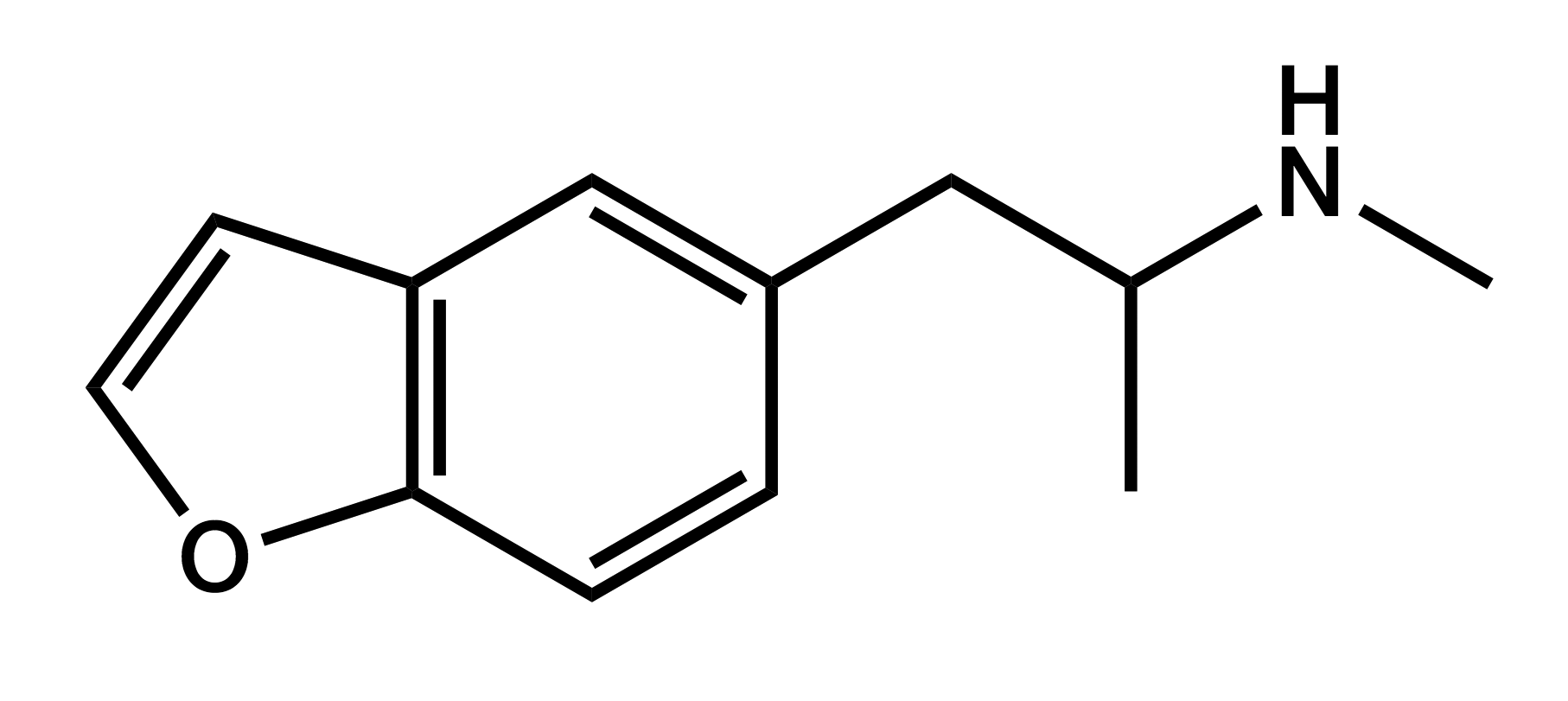
|
It seems to be strongly active at ~100 mg, making it roughly the same potency as MDMA. It is also reportedly more stimulating than either 6-APB or 5-APB.
Sepher commented on Bluelight:
It's the closest RC I've yet found [to MDMA] and it produced very good results in a club. Effects were broadly similar but it's still not quite there. Not quite as full on as far as energy, euphoria and empathy are concerned. You'd probably feel there was something missing if you took it expecting an exact substitute and be disappointed by it. Take it for what it is though. I think it's a very worthwhile substance.
TCB-2
(4-Bromo-3,6-dimethoxy-1,2- dihydrocyclobutabenzene-1-yl)methanamine
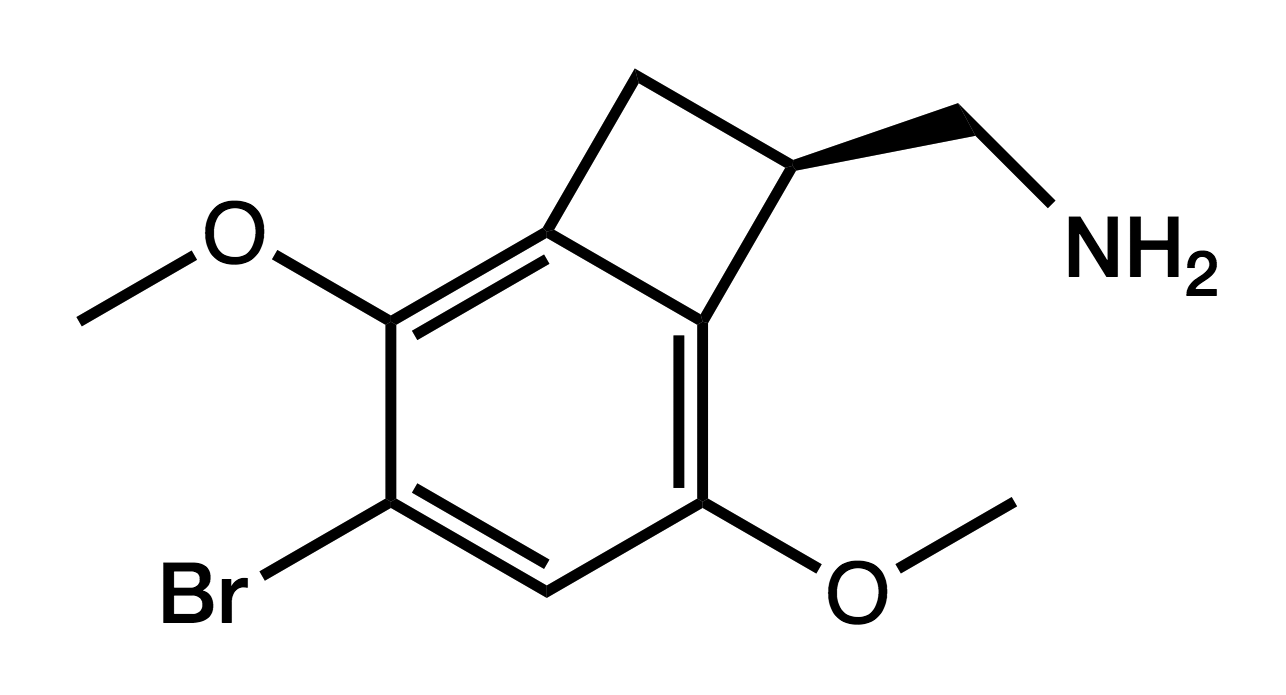
|
In "TCB-2 Overview", published on Erowid in September 2012, BilZ0r describes a series of increasing doses with TCB-2 hydrobromide salt:
T+0:00: 250 µg ingested orally.
T+1:55: Possible alert. Unsure. Nothing else to report. Slept well.
The second day:
T+0:00: 750 µg ingested orally.
T+1:15: Clear alert. I sense the classic body signals I feel when I take hallucinogens, an uncom¬fortable sensation in my neck.
T+2:15: There has been no progression. No visual or thought disturbances.
T+3:15: Body signals dissipate.
The third day:
T+0:00: 3 mg insufflated. As expected, this is quite painful. The pain continues in my sinus for 15 minutes while I resist sneezing or blowing my nose.
T+2:04: First alert.
T+3:04: Nothing greater to report. Wondering if the previous day's effect was imagined. I settle in to
watch a movie.
T+3:34: I smoke a small amount of marijuana (quarter of a bowl). Feel very, very stoned. Having difficulty expressing myself. Realize I am having subtle visual hallucinations when looking at faces. Oddly, no CEVs.
T+5:04: Still having subtle hallucinations and difficulty expressing my thoughts when speaking. This is definitely a mild psychedelic experience.
T+5:34: Take 3.5 mg of zopiclone to sleep. I wake the next day feeling fine.
One week later:
T+0:00: Ingest 5 mg orally with S (a 110 lb female). Begin to make a stew, assuming it would take several hours to kick in as it did previously.
T+0:13: First alert. This is not going to take hours to kick in.
T+0:28: The classic body sensations continue to mount for half an hour. No visuals to report, however I am having significant difficulties in expressing myself succinctly. S reports that she felt it kick in within 15 minutes, but didn't want to say anything after I had claimed, "it will take several hours to have an effect". I timed my dose with the with the assumption that it would be several hours before I felt effects, however, I am already significantly disorientated as I make the bean stew. Thankfully I just get it done before I start to experience coordination difficulties.
T+0:33: Open eye visuals are beginning to start (interestingly, no CEVs). Not currently what I would call classic psychedelic effects, but effects like the TV screen seeming further from me than I know it is, or other size and scale distortions.
T+0:43: Full psychedelic state. ++/+++. Large degree of visual hallucinations, fractals patterns, apparent movement of details, etc.
T+12:45: The ++/+++ state continued for the last 12 hours. I was without anxiety, depression or nausea. S reports similarly. She had a slightly higher degree of visual hallucinations. Insignificant side effects are very shaky hands. S is very stimulated and declares she will not be able to sleep. I am unsure if I will be able to. We both ingest approximately 10 mg of zopiclone to sleep.
T+23:00: The next day, there are still subtle visual effects, like visual snow. We are both tired and groggy. I develop a rather bad headache. S does not. I attribute the headache to dehydration due to general partying over the preceding days. The next day the headache is gone, and I feel fine.
Conclusions: TCB-2 does not appear to be the ultra potent hallucinogen some of its pharmacological properties suggest it should be. It appears to be somewhat less potent than DOI.
Documenting and determining dosage ranges in humans is difficult when only a small number of experience reports are available. This is one of the major issues faced when collecting data on such novel compounds. While BilZ0r speculates that TCB-2 is less potent than DOI (which is active at around a milligram), an author on Bluelight describes TCB-2 as fully active at 250 µg (0.25 mg). Potency extrapolated from drug substitution studies in rats suggests its dosage is on par with LSD or Bromo-Dragonfly.
Pyrazolam
8-Bromo-1-methyl-6-(pyridin-2-yl)-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
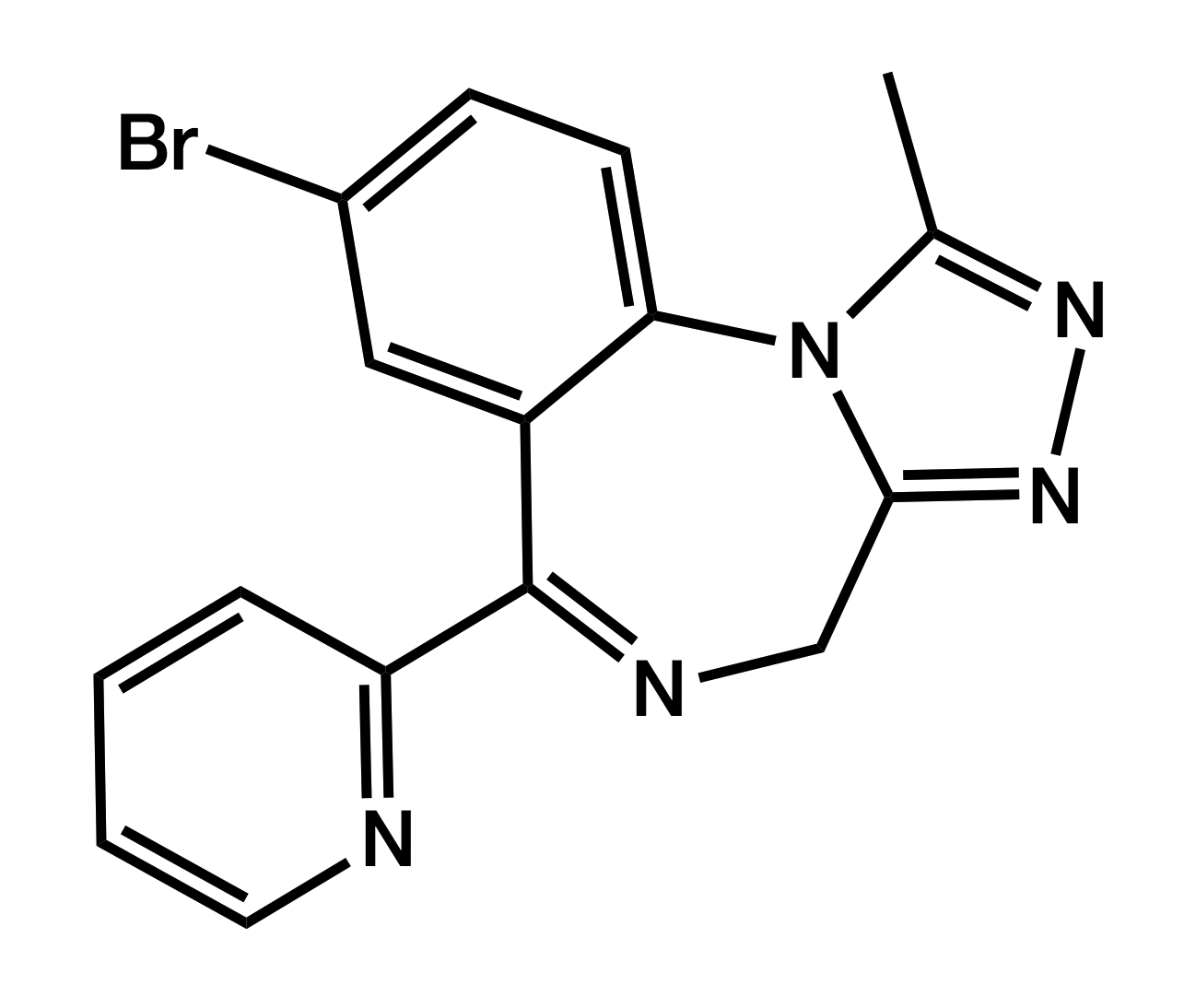
|
Pyrazolam does not seem to be very euphoric; the come-up is pretty drawn out.
Having 3 mg made me pretty intoxicated but if you have a tolerance they don't offer good value.
After trying pyrazolam, I would say it is a lot less euphoric than etizolam and diazepam although it shares the amnesia/blackout/clumsy traits and length of diazepam.
Flubromazepam
7-Bromo-5-(2-fluorophenyl)-1,3-dihydro-1,4-benzodiazepin-2-one
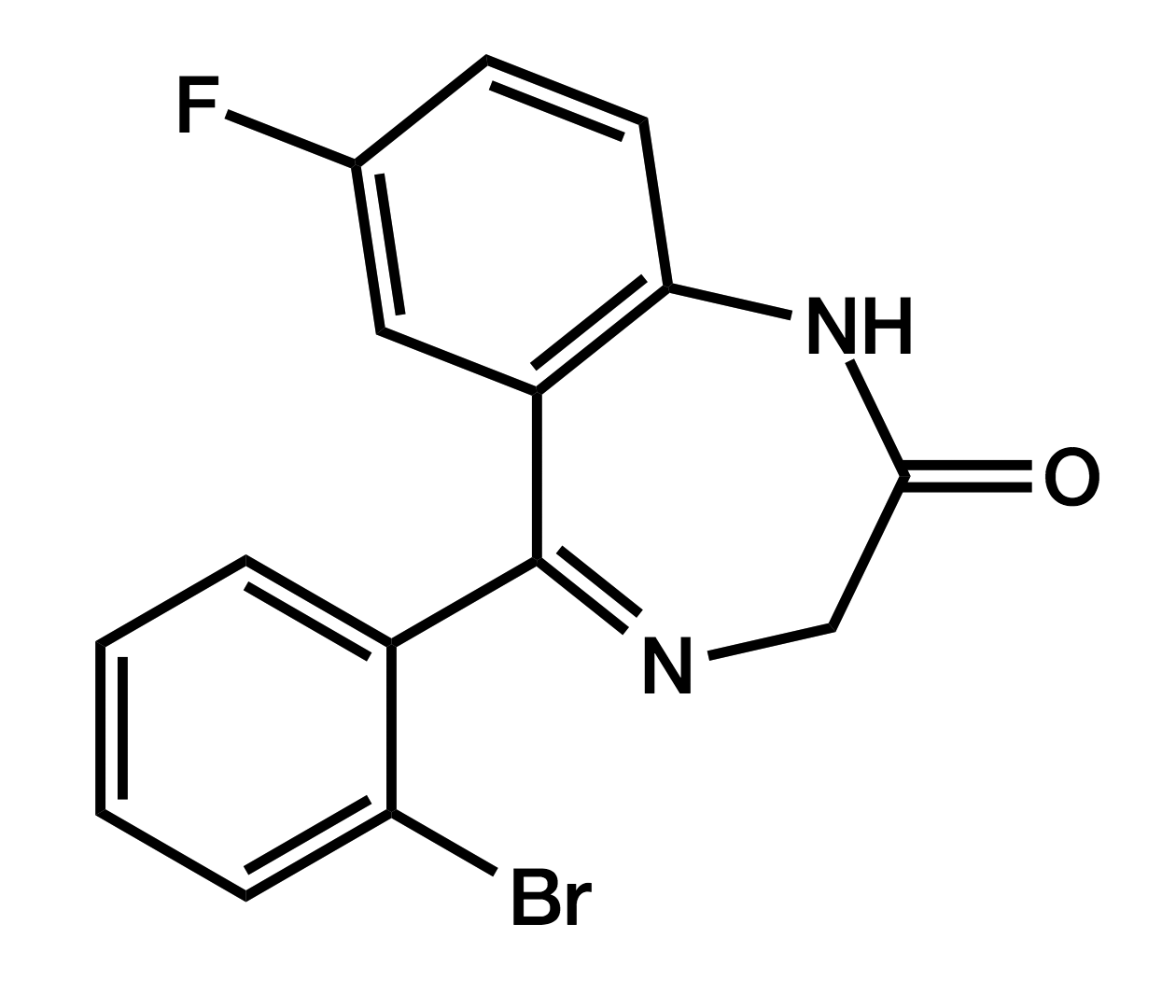
|
Initial reports posted to discussion forums were positive and drew comparisons to clonazepam, diazepam, alprazolam and etizolam. In late 2012, Mela wrote on Bluelight:
8 mg sublingual. 60 minutes later, initial sedative effect developed which evolved into mainly warm chilled anxiolysis by the 3rd hour. A few things happened later which, shall we say, meant it was difficult to distinguish its specific effects. Looks like a winner in the k-pin [klonopin] ballpark (given proposed 30 hour half-life). [...]
The onset effects are more in the diazepam ballpark (notably sedating). I tried 4 mg a few days later, and still found it sedating for an hour or so at this lower dose. For instance, I don't find clonazepam (1 mg), lorazepam (2 mg), alprazolam (1 mg), or even etizolam (at 1 mg) as sedating as 4mg of flubromazepam.
This compound seems likely to see increased use due to it recently becoming more widely available.
Notes #
- "Folkhälsoinstitutet föreslår förbud för ny livsfarlig nätdrog". Statens folkhälsoinstitut. Jul 26, 2012. fhi.se/Aktuellt/Nyheter/Folkhalsoinstitutet-foreslar-forbud-for-ny-livsfarlig-natdrog/
- Seetohul LN, Maskell PD, De Paoli G, et al. "Deaths associated with new designer drug 5-IT". [letter] BMJ. Aug 24, 2012;345:e55625. bmj.com/content/345/bmj.e5625 [This article entry in Erowid References]


