Tryptamine was first synthesized by means of Fischer indole cyclization of the phenylhydrazone of 4-aminobutyraldehyde2. Later, Majima and Hoshino3 prepared it by reduction of indol-3-yl-acetonitrile which was derived from the reaction of indol-3-yl-magnesium iodide with chloroacetonitrile. These methods have been superseded by the preparative sequence involving gramine methiodide4, and by the synthesis via indol-3-yl-glyoxylamides5,6. Other methods are the lithium aluminum hydride reduction of either 3-(2-nitroethyl)-indole7 or 3-(2-nitrovinyl)-indole8. A synthesis by the reaction of indolylmagnesium bromide with ethylene imine was also reported9. However, these methods require expensive materials and numerous steps. Now we wish to report an inexpensive one-step preparation of tryptamine from L-tryptophan (1).
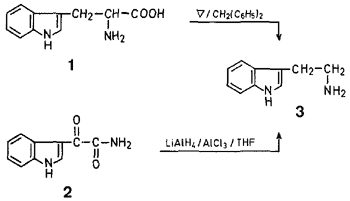
Thus, a suspension of L-tryptophan in forty times of weight of diphenylmethane was gently refluxed under a stream of nitrogen for 15--20 min, during which time evolution of carbon dioxide was observed. After cooling, the clear yellow reaction mixture was treated with hydrogen chloride, and the resulting precipitate was collected and recrystallised to give tryptamine hydrochloride in 60-63 % yield.
Reduction of indol-3-yl-glyoxylamide10,11 (2) with a mixture of aluminum chloride and lithium aluminum hydride (1:1) gave tryptamine hydrochloride in ~70% yield.
Experimental
Tryptamine Hydrochloride from L-Tryptophan (1):
A suspension of L-tryptophan (250 mg) in warm diphenylmethane (10 g) was gently refluxed in a stream of nitrogen for 5-20 min until no more evolution of carbon dioxide was observed. After cooling, the clear pale yellow reaction mixture was treated with a benzene solution (20 ml) saturated with dry hydrogen chloride. The resulting precipitate was collected by filtration, washed with n-hexane and dried to afford crude tryptamine hydrochloride (223 mg, 93%) which was recrystallised from ethanol/ethyl acetate to yield tryptamine hydrochloride (151 mg, 63%) as colorless needles, mp 248-249°C (Ref.2, mp 246-248°C).
Hydride Reduction of Indole-3-yl-glyoxylamide (2):
To a suspension of lithium aluminum hydride (8 g) in tetrahydrofuran (200 ml) was added a solution of anhydrous aluminum chloride (28 g) in tetrahydrofuran (300 ml) dropwise and with ice-cooling during 1.5 hr. To this hydride solution was added dropwise a solution of indol-3-yl-glyoxylamide10,11(8 g) in tetrahydrofuran (400 ml) during 1.5 hr. The reaction mixture was then stirred at 20-25° for 6 hr. An aqueous solution of 30°C sodium hydroxide (400 ml) was added to the above mixture under ice-cooling, and the reaction mixture was filtered off using celite. The filtrate was evaporated under reduced pressure under a current of nitrogen to give a brown residue which was extracted with a mixed solvent ethyl ether/methanol (95:5). Removal of the solvent gave a solid which was dissolved in ethyl ether/methanol (95:5). The resulting solution was then treated with dry hydrogen chloride to form a precipitate. Filtration, followed by evaporation of the filtrate to dryness, afforded tryptamine hydrochloride: yield: 1.5 g (70%). Recrystallization from ethanol/ethyl acetate gave colorless needles, mp 246-248°C.