Isosafrole in acetonitrile + H2O2 in methanol + sodium carbonate (pH 8-10.5), at 10-45°C for 20h gives 94% isosafrole epoxide1. This can be rearranged to MDP2P in about 90% overall yield. Possibly this could also work with straight safrole.
I don't have more info than this, but if I were to perform this reaction, I would try the following procedure:
- Prepare a solution of isosafrole in 5-10 times the volume CH3CN.
- Add a slight molar excess of H2O2 to MeOH (same volume as above), add Na2CO3 until pH is 8-10.5.
- Place the MeOH solution into a water bath and slowly add the CH3CN solution of isosafrole.
- Stir for a day.
- Remove the solvents under vacuum.
- Extract the residue with an organic solvent, wash with water, dry if necessary, and evaporate.
- Dissolve the residue in 10 volumes ethyl acetate, add a few percent LiI or LiBr.
- Reflux for a day.
- Wash the solvent with water to extract the Li salts (recycle), dry solvent, evaporate.
- Residue should be MDP2P, further purification probably a waste of time.
Theory
Radziszewski reaction:
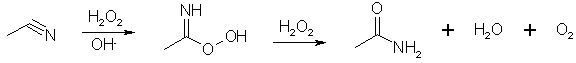
In alkaline solution, H2O2 reduces the intermediate peroxy acid to the amide with the liberation of oxygen, but at pH 8 no oxygen is evolved and the solution of peroxycarboximidic acid can be used to oxidize an olefin to its epoxide2. Too much hydrogen peroxide oxidizes the acetonitrile to acetamide.
A low-cost method for epoxidation of cyclohexene is by dropwise addition of 30% H2O2 and NaOH to a stirred mixture of cyclohexene, methanol, and acetonitrile at 60°C, the yield of cyclohexene oxide is 85%3.
Dimethyldioxirane Epoxidation of anethole and asarone4
Dimethyldioxirane solution in acetone was made freshly by an improved conventional method5. After the solution was dried with molecular sieves (4 Å, activated powder), it was titrated iodometically with KI and starch. The titrated dimethyldioxirane solution (~0.08 M solution in acetone, 1.2 equiv.) was added to trans-anethole or trans-asarone in dry acetone (1 ml for 1 mmol) at 0°C and the reaction mixture was stirred for 30 min at room temperature. The reaction solution was evaporated under reduced pressure to afford the epoxides. trans-anethole oxide was a colorless oil. The yellow oil of trans-asarone oxide after vacuum application was dissolved in dry hexane and recrystallized at –20°C for a few days to produce a white solid product (mp. 37–40°C). The yields of these oxides were >95%. The oxides were kept at –80°C in dry nitrogen. In this condition trans-anethole oxide was stable for 1 year and trans-asarone oxide was stable for 1 month.
Purity of trans-anethole oxide and trans-asarone oxide
trans-anethole oxide and trans-asarone oxide were found to have very pure NMR spectra with little or no impurity peaks. The yields of oxides from this method were >95%, which are much better than those of Mohan and Whalen6 and Greca et al.7 whose methods used m-chloroperoxybenzoic acid to oxidize anethole or asarone and gave yields of 38 and 52%, respectively.
Stability of trans-anethole oxide and trans-asarone oxide
Both of the oxides were stable in acetone or DMSO for 1 h at 0 or 37°C. In aqueous environments, however, the amount of these epoxides declined, presumably because of their hydration to diols. The half-life of trans-anethole oxide was 7.6 min in 0.1 M potassium phosphate buffer (pH 7.4) at 37°C. The presence of 154 mM KCl lowered the half-life to 4.2 min. trans-Asarone oxide showed shorter half-lives; 4.0 min without or 2.4 min with 154 mM KCl.
trans-Anethole Oxide8
To a well-stirred biphasic mixture of 2.0 g of trans-anethole in 60 mL of methylene chloride and 60 mL of 10% sodium carbonate in water in an icewater bath was added a solution of 5.6 g of m-chloroperoxybenzoic acid (85%, 0.028 mol) in 60 mL of methylene chloride over a period of 2.5 h by means of a syringe pump assembly. The methylene chloride layer was separated, washed with 10% sodium carbonate solution (3x25 mL) and saturated sodium chloride solution (25 mL), and dried over anhydrous sodium sulfate. Removal of the solvent on a rotary evaporator yielded 1.94 g of oil that was distilled in a short path distillation apparatus (45°C oil bath, 0.1 mmHg) to yield 0.84 g (38%) of trans-Anethole Oxide.