Abstract
Synthesis of (1S,2S)-pseudoephedrine is described via reduction of an alanine-derived oxazolidinone followed by treating with phenyl magnesium bromide and chemoselective N-methylation in an efficient and practical manner.
In recent years there has been considerable interest in the synthesis of optically pure amino alcohols because of their wide occurrence in several biologically active molecules and also their application as chiral building blocks, auxiliaries and ligands in asymmetric synthesis1,2. Among this class, ephedrine and pseudoephedrine have found wide application for a variety of purposes in organic synthesis. Pseudoephedrine, especially, has been used extensively in asymmetric alkylations of corresponding carboxamides3,4. Recently, we have reported chemoselective reduction of N-protected oxazolidinones to the corresponding lactol derivatives and their application in the synthesis of vicinal amino alcohols5. In this paper, we wish to report a new approach for the synthesis of (1S,2S)-pseudoephedrine by using the synthetic potential of our methodology. The key steps involved in the present synthesis are: (i) reduction of the N-Cbz oxazolidinone derived from alanine (2 to 3); (ii) Grignard addition to the lactol to give the amino alcohol (3 to 4); and (iii) chemoselective N-methylation (4 to 8) as shown in Scheme 1.
Scheme 1
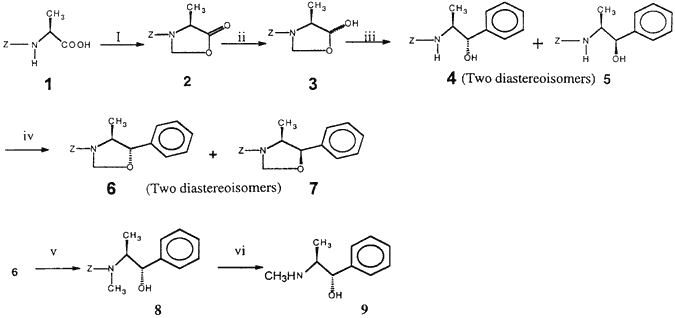
Reagents and conditions:
(i) (CH2O)n, PTSA, benzene, reflux, 1 h; (ii) 1 equiv. NaBH4, MeOH, 2 h; (iii) PhMgBr, THF, 0°C–rt, 3 h;
(iv) (CH2O)n, PTSA, benzene, reflux, 1 h; (v) NaCNBH3/TMSCl, CH3CN, rt, 30 min; (vi) conc. HCl, reflux, 2 h
The oxazolidinone (2)6, readily obtained from N-Cbz-L-alanine (1) by reacting with paraformaldehyde in the presence of catalytic PTSA, was treated with 1 equiv. of sodium borohydride to give the lactol 3 in excellent yield (95%) as a colourless syrup [α]D -2° (c 1, MeOH). Treatment of lactol (3) with phenyl magnesium bromide afforded an inseparable diastereomeric mixture (4 and 5) in 92% combined yield, ratio 95:5 from 1H-NMR. The newly created stereocentre in the major isomer 4 was initially assumed to be S by analogy with our earlier related work5, and was confirmed by conversion into the known title compound. To circumvent the separation problem, the diastereomeric mixture was reacted with paraformaldehyde in the presence of cat. PTSA to furnish oxazolines 6 and 7 in a combined yield of 96%. Oxazoline 6 was easily separated from 7 by using column chromatography in 94% yield (based on crude 6 and 7) as a colourless syrup, [α]D -21.5° (c 1, MeOH). Treatment of oxazoline 6 with NaCNBH3/TMSCl smoothly afforded the N-methyl amino alcohol 8 in 93% yield. Acidic hydrolysis of 8 afforded (1S,2S)-pseudoephedrine hydrochloride as white crystalline solid in 92% yield. M.p. 184–186°C, [α]D +59.8° (c 2, H2O) lit7 m.p. 185–188, [α]D +61° (c 1, H2O), thereby confirming the structure assignment and configurations.
In summary, we have demonstrated an efficient new synthesis of pseudoephedrine in excellent yield. This synthesis will be amenable to the syntheses of analogous compounds with ease. Further work is in progress and will be reported in due course.