Synthesis of Piperonalvia chloromethylation and reaction with sodium 2-propanenitronate[ Back to the Chemistry Archive ] Chloromethylation of methylenedioxybenzene (1,3-benzodioxole) to piperonylchloride (3,4-methylenedioxybenzyl chloride), followed by reaction with the sodium salt of 2-nitropropane in an alcoholic solvent can be used to synthesize piperonal. The reaction is general and can be applied to the synthesis of other substituted benzaldehydes. In the first example they use ethanol as solvent for the second step, and in example 2 aqueous isopropanol is used. Aqueous tert-Butyl alcohol can also be used with a small increase in yield, but not enough to warrant the higher price of that solvent. 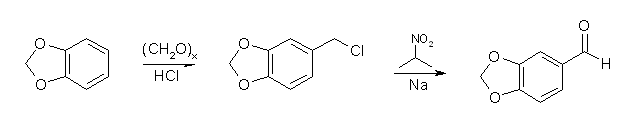
Example 1(a) Paraformaldehyde (3.0 g, 0.1 mole) was suspended in cold (0°C) aqueous hydrogen chloride (20 ml) that had been saturated at 0°C. Methylenedioxybenzene (12.2 g, 0.1 mole) was added and the reaction mixture was stirred at 0°C for 4 hours. The oil was separated, the aqueous layer extracted three times with methylene chloride (10 ml portions), the total organic layers dried with MgSO4, and the solvent removed in vacuo. (b) To a solution of sodium (1.84 g, 0.08 mole) in ethanol (60 ml) was added 2-nitropropane (7.12 g; 0.08 mole) to obtain a thick but stirrable white slurry. The chloromethyl mixture prepared as in (a) was added dropwise over a few minutes, the reaction mixture becoming bright orange in color, this color fading rapidly once the addition had been completed. The reaction mixture was stirred at room temperature for 64 hours over which time it became much thinner in consistency. The pH was adjusted from about 9.0 to about 6.0 by the addition of a little HCl, and the reaction mixture was subjected to steam distillation, collecting first the ethanol, then water-containing Piperonal. Distillation was continued until the distillate no longer gave a positive reaction with 2,4-dinitrophenylhydrazine, some 3.5 liters of distillate being collected. The distillate was saturated with sodium chloride, extracted three times with methylene chloride, the combined extracts dried with MgSO4 and evaporated to leave 9.5 g residue. The ethanol initially separated was evaporated to dryness at room temperature in vacuo to yield a further 0.2 g. Distillation at 15 mm produced a 1:1 mixture of acetoxime and Methylenedioxybenzene (0.5 g), b.p. 26-46°C/15 mmHg, a further 2.5 g of Methylenedioxybenzene, b.p. 60-65°C/15 mm, and finally 5.85 g, b.p. 128-132°C/15 mm of Piperonal together with a small amount of Piperonyl ethyl ether. The yield of crude Piperonal was 55% based on unrecovered methylenedioxybenzene. Example 2(a) Paraformaldehyde (4.5 g, 0.15 mole) was suspended at 0°C in saturated aqueous hydrogen chloride (20 ml). Methylenedioxybenzene (12.2 g, 0.1 mole) was added and the reaction mixture stirred between 17-20°C with a slow stream of hydrogen chloride passing through the mixture. Stirring was continued for 1.5 hours when almost all the methylenedioxybenzene had disappeared. The mixture, composed mainly of Piperonyl chloride together with some bis-chloromethyl-methylenedioxybenzene and methylenedioxybenzene was separated, the aqueous layer extracted twice with 10 ml portions of methylene chloride and the total organic layer evaporated at 30-35°C in vacuo. (b) To a stirred mixture of water (5 ml) and 2-nitropropane (8.9 g, 0.1 mole) at 20°C was added a solution of sodium hydroxide (4.1 g, 0.1 mole, 98% purity) in water (5.0 ml) over 2-3 minutes and the mixture was stirred at 20-30°C for 60 minutes during which time it became homogeneous. Isopropyl alcohol (10 ml) was added, followed by the chloromethylated mixture prepared as in (a), the transfer being completed by use of isopropyl alcohol (2 ml). Sodium hydroxide (0.1 g) was added to ensure neutralization of any hydrogen chloride, then the whole was stirred rapidly on a bath at 35-40°C. The reaction mixture thinned rapidly and an exotherm (7-8°C above the oil bath temperature) ceased after 45 min. The oil bath temperature was raised to 50°C and after a total time of 2.5 hours GLC analysis indicated virtual completion of reaction. The reaction mixture was pumped in vacuo to remove volatile material at 80°C, first at 15 mm, then at 30°C and 0.5 mm. The residue was stirred rapidly with sodium metabisulphite (10 g) and water (12 ml). Further addition of metabisulphite (5 g) and water (15 ml) ensured completion of the bisulphite addition reaction after 2.5 hours. Ethanol (20 ml) was then added, the whole filtered and the solid washed with ethanol (3x25 ml) and methylene chloride (2x25 ml). The solid was suspended in water (50 ml) and methylene chloride (30 ml) and decomposed with saturated sodium carbonate solution below pH 8.5. The layers were separated, the aqueous layer extracted with methylene chloride and the total organic solution dried with MgSO4, decolorized with charcoal and evaporated in vacuo to give 7.0 g crude piperonal; yeild 46% on total methylenedioxybenzene. References[1] British Pat 1,538,214 |