In 1991, Alexander (Sasha) and Ann Shulgin published what would have to be the greatest single contribution to pychedelic chemistry, ever. I refer, of course, to PiHKAL, or Phenethylamines I Have Known And Loved. The hefty 978-page tome is actually two books bound together. Book I, presented in three parts, is a fascinating and intimate look into the lives Shura and Alice Borodin, two fictional characters who bear more than a passing resemblance to Sasha and Ann Shulgin. Book II is a compilation of syntheses, "trip reports" and notes regarding 179 substances synthesized, in most cases for the first time, by Sasha Shulgin.
Book II was made available online with the permission of the Shuglins, to faciltate the free spread of this information. It is currently available at Erowid and at The Lycaeum, which also has all of the structures online. However, it is worth noting that the Shuglins deserve our support, especially since the U.S. government began persecuting them in 1994. Buy this book if you can spare the cash (US $18.95), preferably from Mind Books, who also deserve our support.
With such a large inventory of molecules, the naming scheme is necessarily somewhat esoteric. For the casual reader with no formal chemistry training, such as myself, it is apparent on reading PiKHAL that there is some underlying plan to the collection of substances Shulgin has synthesized, however the specifics of this are quite elusive. For this reason, primarily for my own education, I decided to prepare a kind of "tour" of the substances presented in PiHKAL, outlining the structural relationships and naming schemes. I thought this could be of value to others so I've written it up in the form of this document.
For improved readability you may wish to resize the browser until it is just wide enough to lose its horizontal scrollbar.
As I noted above, my level of chemistry knowledge is not great, however I hope it is sufficient for the purposes of this tour. For those with no chemsitry knowledge, I present here a quick rundown of what you need to know. Some people people may wish to skip to the bit where the phenethylamine backbone is introduced, or even skip straight to the start of the tour.
First of all, all of the materials in PiHKAL are known as compounds. This means that they are made up of innumerable tiny components known as molecules, and that said molecules are all identical and are made up of a number of different atoms. Atoms themselves are made up of a nucleus, which contains protons and neutrons, and the nucleus is surrounded by a cloud of electrons. Phew, talk about reductionism. For the purposes of chemistry, protons, electrons and neutrons can be considered to be indivisible. Electrons and protons are attracted to each-other, which is why the electrons cloud around the nucleus, but they can't get too close or they start being repelled. The esoteric laws of Quantum mechanics end up dictating that there is a series of allowed energies of electrons in atoms, and that only a certain number of electrons can occupy a given level. This number increases with the energy of the level. The normal state of an atom is that all its levels, except perhaps one uppermost layer, are filled, and that the number of electrons is equal to the number of protons in the nucleus. Atoms are given names according to the number of protons : one proton is hydrogen, two protons is helium, three is lithium, etc : these are called the elements. Chemistry, by and large, is about the interactions of atoms, and these interactions usually have something to do with the electrons in the upper-most occupied energy level. This means that in many situations, different elements may behave in similar ways in chemical reactions, if they share the same number of electrons, or same number of free spaces, in their outer shells. The Periodic Table of the Elements provides a graphical representation of this, which we won't go into too deeply here.
The most important elements in psychdelic chemistry are carbon, hydrogen and nitrogen. In organic molecules the atoms are joined together with a type of bond known as the covalent bond. In covalent bonding, the atoms more or less share the electrons of their outer shells in order to fill them completely. Since hydrogen has one electron and one free space, two hydrogen atoms can bond to form molecular hydrogen, written as H2 (where the subscript "2" means the molecule has two of these things : this is called an empirical formula). Carbon has four electrons and four empty slots in its outer level, so it can bind to four hydrogen atoms to form CH4, known as methane. Two carbon atoms could also share a pair of electrons, leaving three free slots in each, which we could fill with three hydrogen atoms each in a similar way to what we did with methane, to form C2H6, or ethane. Alternatively, we could share two pairs of electrons, in what is known as a double bond, meaning only two hydrogens are needed for each carbon, to form C2H4, or ethene : the -ene signifies the presence of a double bond. Or, we could connect together three carbon atoms with two single bonds, requiring three hydrogens for each of the two end carbons, and two hydrogens for the middle one: this is C3H6, or propane. If we keep adding atoms of single-bonded carbon and hydrogen, we form butane, pentane, hexane, heptane, octane, and so on. This is called a homologous series. For members of the methane series (the saturated (all single bonds) aliphatic (in chains) hydrocarbons) above propane, one can find several arrangements (called isomers) of each collection of atoms : for example, an isomer of pentane could be made with a central carbon, singly linked to four -CH3 (methyl) groups. This could be called, ambiguously, isopentane, or more precisely, 2,2-dimethylpropane, that is propane with two methyl groups (-CH3s) attached to the 2nd carbon.
We could go on forever making ever more complex organic molecules: this is the beauty of carbon. Organic chemistry is like playing with Lego. However, these simple hydrocarbons don't make good drugs, unless you want brain damage, so let's move on. The next thing you need to know about is the benzene ring. Take a CH and singly bond its carbon to the carbon of another CH. Then doubly bond this carbon to the carbon of another CH. Then singly bond that carbon to another CH, doubly bond that carbon to the carbon of another CH, singly bond that carbon to the carbon of another CH, and doubly bond that carbon to the carbon of the original CH. That was a mouthful, from now on we will simply draw the molecules, like this: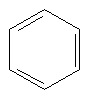
Here, each line represents a covalent bond, and each vertex represents a carbon atom. To keep things simple, hydrogen atoms are often left out of pictures of structures, since it is easy to work out where they must go. In this case we know that carbon always wants four bonds, so here each carbon must have one hydrogen hanging off it. Now, it actually turns out that the benzene ring is so small that all of the electrons in the depicted single and double bonds are in fact shared equally among the carbons, so the benzene ring is often drawn like this:
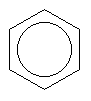
Now, remember before when named a pentane isomer by viewing it as propane with two methyl groups attached? This kind of thing happens quite a bit in organic chemistry, where we can replace one chunk of molecule (in this case a -H) with another (in this case -CH3) that has the same bonding requirements. The chunks are called functional groups, and are often referred to as simply "R", when "R" could be any of several function groups leading to a variety of different molecules based on a common backbone. As you may have surmised, functional groups have names which are often derived from molecules that consist of the functional group plus one or more hydrogens. Hence, -CH3 is refered to as methyl (often shortened to Me). We could also make use of ethyl (-C2H5, Et), propyl, and so-on. Or, we could put an oxygen (which wants to have two bonds) between the group and whatever it is to be tacked on to, to have hydroxy (-OH), methoxy (-OCH3 or MeO), ethoxy, and so-on. Or we could pull the same trick with a sulfur atom to make methylthio (-MeS), ethylthio (-EtS) and so-on.
One very imporant functional group is the amine (NH2) group. This is based on ammonia, (NH3), and is a feature of the vast majority of psychoactive substances. If we were to take a benzene ring, use it as a functional group (called phenyl) to tack on to an ethyl group, and tack on an amine on the other end of the ethyl, we would have beta-phenethylamine, like so:

This molecule is the basis of all of the substances described in book
II of PiHKAL. In order to talk about modifications to the molecule, it
is useful to have a way of referring to each carbon atom. Starting
with the benzene ring at the site of the bond to the ethyl, we number
this "1", and travelling in either direction, number the rest 2, 3, 4,
5, 6. Note that one could just as easily have looked at the structure
from the other side (imagine yourself inside the computer monitor or
behind the page) : this is why the 2-position could also be called the
6-position, as long as what was the 3-position was referred to as the
5-position. The other two carbons are called ![]() (alpha) and
(alpha) and ![]() (beta), starting from the right. The
most interesting compounds are those with the phenyl attached at the
beta position (a la beta-phenethylamine). Some alpha-phenethylamines
are discussed in the MDA entry of PiHKAL.
(beta), starting from the right. The
most interesting compounds are those with the phenyl attached at the
beta position (a la beta-phenethylamine). Some alpha-phenethylamines
are discussed in the MDA entry of PiHKAL.
Phenethylamine itself is not active![]() -position : this gives us
amphetamine:
-position : this gives us
amphetamine:
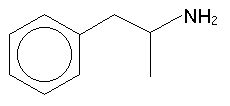
Adding or removing an alpha-methyl is a commonly used tactic in structural explorations. Shulgin refers to the alpha-methylated molecule as the three-carbon or 3C- version (or the "amphetamine analogue"), and naturally, when the alpha-methyl is not present, the substance is the two-carbon, 2C- or "phenethylamine analogue". Amphetamine is an interesting substance however it is not a psychedelic.
Another substance worth knowing about is dopamine, since it is a neurotransmitter that is intimately involved (along with serotonin, a tryptamine) in the action of the phenethylamines, among many other drugs. Dopamine is 3,4-dihydroxyphenthylamine (the di- means two hydroxys):
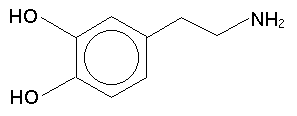
Shulgin employed a wide range of functional groups in his creations and it isn't worth bogging ourselves down with the structure of each here: you already have the most common ones. You might refer to an organic chemistry textbook, or perhaps you could even find something at Web-ster's Organic Chemistry Site, if you are interested.
What follows is a tour of the substances described in PiHKAL. When discussing variations to structures, it will be convenient to talk as if we are able to just add groups, atoms, even neutrons wherever we like. Of course in real life this is not the case and a small change in structure will sometimes demand an entirely different synthesis strategy.
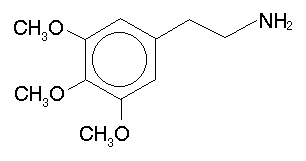
The most obvious thing to try is to methylate that alpha carbon, turning the phenethylamine bit into amphetamine. The result in this case is 3,4,5-trimethoxyamphetamine, or simply TMA. One could also move those methoxys around to different positions (there are six possibilities), forming both the phenethylamine (PEA) and amphetamine (A) versions. The following table summarizes these substances:
| MeO @ | -A | -PEA |
| 3,4,5 | TMA | mescaline |
| 2,4,5 | TMA-2 | TMPEA |
| 2,3,4 | TMA-3 | IM |
| 2,3,5 | TMA-4 | untested |
| 2,3,6 | TMA-5 | untested |
| 2,4,6 | TMA-6 | untested |
| 4-R | -PEA | -A |
| MeO | mescaline | TMA |
| trideuteromethoxy | 4-D | |
| Me | DESOXY | |
| Br | 4-Br-3,5-DMA | |
| EtO | E | |
| isopropoxy | IP | |
| propoxy | P | |
| cyclopropylmethoxy | CPM | |
| butoxy | B | |
| benzyloxy | 3C-BZ | |
| phenethyloxy | PE | |
| allyloxy | AL | |
| methallyloxy | MAL | |
| propynyloxy | PROPYNYL | |
| methylthio | 4-TM | |
| ethylthio | 4-TE | |
| butylthio |
TB | |
| propylthio | TP |
Starting from the top of the table we have, of course, mescaline. This is followed by 4-D, in which the three hydrogen atoms of the 4-methoxy group are present as the single neutron isotope, deuterium. Next is DESOXY, so-named since it is mescaline with the oxygen removed from the 4-methoxy. This is followed by a series of increasingly massive hydrocarbon substituents, a number of which also have alkylthio versions included at the end of the table. The 3,4-dimethoxy-5-alkoxy versions of E and P are also included in PiHKAL, as ME and MP -- the "M" stands for "meta".
Shulgin notes in the entry for PE, and it is worth repeating here, that 3C-BZ (and its untasted phenethylamine version, BZ) has nothing to do with the agent of chemical warfare also known as BZ (thankfully).
We have already seen escaline (E -- unlike the the common usage of the term, which is short for Ecstacy, which is supposedly MDMA) in the above section. A number of analogues are possible by replacing combinations of the 3,4,5-methoxys with an ethoxy. These are summarized in the following table. The strange naming of SB refers to the fact that it is 3,5-bisescaline and that the ethoxys are symmetrically placed on the ring. ASB is the asymmetric version; it is this PiHKAL entry in which Shulgin describes the naming. In chemical nomenclature the "s" in "bis" means, to paraphrase from Shulgin in the ASB entry, that there are two somethings (ethoxys) attached to something (phenethylamine), rather than three whatevers (biescaline, two escalines). Likewise, TRIS is 3,4,5-triethoxyphenethylamine, or 3,4,5-trisescaline.
Analogues of these have also been made by replacing one or more oxygen (as part of a methoxy or ethoxy) with a sulfur atom. The following table lists the relevant substances to be found in PiHKAL:
| EtO @ | -A | 3-EtS-A | 4-EtS-A | 5-EtS-A |
| 4 | E | 3-TE | 4-TE | N/A |
| 3 | ME |
3-TME | 4-TME | 5-TME |
| 3,5 | SB | 3-TSB | 4-TSB | |
| 3,4 | ASB | 3-TASB | 4-TASB | 5-TASB |
| 3,4,5 | TRIS | 3-TRIS | 4-TRIS |
The methylthio analogues of mescaline and IM (isomescaline) have their own entries: 3-TM and 4-TM, and 2-TIM, 3-TIM and 4-TIM.
Two other derivatives of mescaline are included in PiHKAL: The addition of neutrons to the beta-hydrogens (making them deuteriums) gives beta-D, and addition of a methoxy at the beta position gives BOM.
The exploration of the TMA isomers led to the discovery of the magic of the 2,4,5 configuration. As described above, the inspiration for this was mescaline, however the family tree of the 2,4,5-s is so rich that it deserves its own section.
| 4-R | -A | -A, 2-MeS | -A, 5-MeS | -PEA | |
| H | 2,5-DMA | 2C-H | |||
| Me | DOM | 2-TOM | 5-TOM | 2C-B | MeO : BOD; OH : BOHD |
| Et | DOET | 2-TOET | 5-TOET | 2C-E | |
| propyl | DOPR | 2C-P | |||
| butyl | DOBU | ||||
| amyl | DOAM | ||||
| ethylfluoro | DOEF | ||||
| fluoro | DOF; see 2C-F | 2C-F | |||
| chloro | DOC | 2C-C | |||
| bromo | DOB | 2C-B | MeO : BOB | ||
| iodo | DOI | 2C-I | |||
| nitro | DON | 2C-N | |||
| methoxy | TMA-2 | TMPEA | |||
| ethoxy | MEM | ||||
| propoxy | MPM | ||||
| isopropoxy | 2C-O-4 | ||||
| MeSe | 2C-SE | ||||
| MeS | ALEPH | 2C-T | |||
| EtS | ALEPH-2 | 2C-T-2 | |||
| isopropylthio | ALEPH-4 | 2C-T-4 | |||
| phenylthio | ALEPH-6 | ||||
| propylthio | ALEPH-7 | 2C-T-7 | |||
| cyclopropylmethylthio | 2C-T-8 | ||||
| butylthio | 2C-T-9 | ||||
| methoxyethylthio | 2C-T-13 | ||||
| cyclopropylthio | 2C-T-15 | ||||
| butylthio | 2C-T-17 | ||||
| 2-fluoroethylthio | 2C-T-21 |

| N-R | -A |
| H | MDA |
| OH | MDOH |
| MeO | MDMEO |
| Me | MDMA |
| Et | MDE |
| 2-OH-Et | MDHOET |
| 2-MeO-Et | MDMEOT |
| propyl | MDPR |
| isopropyl | MDIP |
| butyl | MDBU |
| benzyl | MDBZ |
| cyclopropylmethyl | MDCPM |
| allyl | MDAL |
| propargyl | MDPL |
| MeO @ | -A |
| 5 | MMDA |
| 6 | MMDA-2 |
| 2 | MMDA-3a |
| 2,5 | DMMDA |
| 5,6 | DMMDA-2 |
| 1 | AEM | 2.1.4 | Erowid | Lycaeum | a-Ethyl-3,4,5-trimethoxy-PEA |
| 2 | AL | 2.1.2 | Erowid | Lycaeum | 4-Allyloxy-3,5-dimethoxy-PEA |
| 3 | ALEPH | 2.2.2 | Erowid | Lycaeum | 4-Methylthio-2,5-dimethoxy-A |
| 4 | ALEPH-2 | 2.2.2 | Erowid | Lycaeum | 4-Ethylthio-2,5-dimethoxy-A |
| 5 | ALEPH-4 | 2.2.2 | Erowid | Lycaeum | 4-Isopropylthio-2,5-dimethoxy-A |
| 6 | ALEPH-6 | 2.2.2 | Erowid | Lycaeum | 4-Phenylthio-2,5-dimethoxy-A |
| 7 | ALEPH-7 | 2.2.2 | Erowid | Lycaeum | 4-Propylthio-2,5-dimethoxy-A |
| 8 | ARIADNE | 2.2.3 | Erowid | Lycaeum | 2,5-Dimethoxy-a-ethyl-4-methyl-PEA |
| 9 | ASB | 2.1.3 | Erowid | Lycaeum | 3,4-Diethoxy-5-methoxy-PEA |
| 10 | B | 2.1.2 | Erowid | Lycaeum | 4-Butoxy-3,5-dimethoxy-PEA |
| 11 | BEATRICE | 2.2.3 | Erowid | Lycaeum | 2,5-Dimethoxy-4,N-dimethyl-A |
| 12 | BIS-TOM | 2.2.2 | Erowid | Lycaeum | 2,5-Bismethylthio-4-methyl-A |
| 13 | BOB | 2.2.2 | Erowid | Lycaeum | 4-Bromo-2,5,beta-trimethoxy-PEA |
| 14 | BOD | 2.2.2 | Erowid | Lycaeum | 2,5,beta-Trimethoxy-4-methyl-PEA |
| 15 | BOH | 2.4.1 | Erowid | Lycaeum | beta-Methoxy-3,4-methylenedioxy-PEA |
| 16 | BOHD | 2.2.2 | Erowid | Lycaeum | 2,5-Dimethoxy-beta-hydroxy-4-methyl-PEA |
| 17 | BOM | 2.1.4 | Erowid | Lycaeum | 3,4,5,beta-Tetramethoxy-PEA |
| 18 | 4-Br-3,5-DMA | 2.1.2 | Erowid | Lycaeum | 4-Bromo-3,5-dimethoxy-A |
| 19 | 2-Br-4,5-MDA | 2.3.5 | Erowid | Lycaeum | 2-Bromo-4,5-methylenedioxy-A |
| 20 | 2C-B | 2.2.2 | Erowid | Lycaeum | 4-Bromo-2,5-dimethoxy-PEA |
| 21 | 3C-BZ | 2.1.2 | Erowid | Lycaeum | 4-Benzyloxy-3,5-dimethoxy-A |
| 22 | 2C-C | 2.2.2 | Erowid | Lycaeum | 4-Chloro-2,5-dimethoxy-PEA |
| 23 | 2C-D | 2.2.2 | Erowid | Lycaeum | 4-Methyl-2,5-dimethoxy-PEA |
| 24 | 2C-E | 2.2.2 | Erowid | Lycaeum | 4-Ethyl-2,5-dimethoxy-PEA |
| 25 | 3C-E | 2.1.3 | Erowid | Lycaeum | 4-Ethoxy-3,5-dimethoxy-A |
| 26 | 2C-F | 2.2.2 | Erowid | Lycaeum | 4-Fluoro-2,5-dimethoxy-PEA |
| 27 | 2C-G | 2.2.3 | Erowid | Lycaeum | 3,4-Dimethyl-2,5-dimethoxy-PEA |
| 28 | 2C-G-3 | 2.2.3 | Erowid | Lycaeum | 3,4-Trimethylene-2,5-dimethoxy-PEA |
| 29 | 2C-G-4 | 2.2.3 | Erowid | Lycaeum | 3,4-Tetramethylene-2,5-dimethoxy-PEA |
| 30 | 2C-G-5 | 2.2.3 | Erowid | Lycaeum | 3,4-Norbornyl-2,5-dimethoxy-PEA |
| 31 | 2C-G-N | 2.2.3 | Erowid | Lycaeum | 1,4-Dimethoxynaphthyl-2-ethylamine |
| 32 | 2C-H | 2.2.2 | Erowid | Lycaeum | 2,5-Dimethoxy-PEA |
| 33 | 2C-I | 2.2.2 | Erowid | Lycaeum | 4-Iodo-2,5-dimethoxy-PEA |
| 34 | 2C-N | 2.2.2 | Erowid | Lycaeum | 4-Nitro-2,5-dimethoxy-PEA |
| 35 | 2C-O-4 | 2.2.2 | Erowid | Lycaeum | 4-Isopropoxy-2,5-dimethoxy-PEA |
| 36 | 2C-P | 2.2.2 | Erowid | Lycaeum | 4-Propyl-2,5-dimethoxy-PEA |
| 37 | CPM | 2.1.2 | Erowid | Lycaeum | 4-Cyclopropylmethoxy-3,5-dimethoxy-PEA |
| 38 | 2C-SE | 2.2.2 | Erowid | Lycaeum | 4-Methylseleno-2,5-dimethoxy-PEA |
| 39 | 2C-T | 2.2.2 | Erowid | Lycaeum | 4-Methylthio-2,5-dimethoxy-PEA |
| 40 | 2C-T-2 | 2.2.2 | Erowid | Lycaeum | 4-Ethylthio-2,5-dimethoxy-PEA |
| 41 | 2C-T-4 | 2.2.2 | Erowid | Lycaeum | 4-Isopropylthio-2,5-dimethoxy-PEA |
| 42 | 2.2.2 | Erowid | Lycaeum | 4-Isopropylthio-2,6-dimethoxy-PEA | |
| 43 | 2C-T-7 | 2.2.2 | Erowid | Lycaeum | 4-Propylthio-2,5-dimethoxy-PEA |
| 44 | 2C-T-8 | 2.2.2 | Erowid | Lycaeum | 4-Cyclopropylmethylthio-2,5-dimethoxy-PEA |
| 45 | 2C-T-9 | 2.2.2 | Erowid | Lycaeum | 4-(t)-Butylthio-2,5-dimethoxy-PEA |
| 46 | 2C-T-13 | 2.2.2 | Erowid | Lycaeum | 4-(2-Methoxyethylthio)-2,5-dimethoxy-PEA |
| 47 | 2C-T-15 | 2.2.2 | Erowid | Lycaeum | 4-Cyclopropylthio-2,5-dimethoxy-PEA |
| 48 | 2C-T-17 | 2.2.2 | Erowid | Lycaeum | 4-(s)-Butylthio-2,5-dimethoxy-PEA |
| 49 | 2C-T-21 | 2.2.2 | Erowid | Lycaeum | 4-(2-Fluoroethylthio)-2,5-dimethoxy-PEA |
| 50 | 4-D | 2.1.1 | Erowid | Lycaeum | 4-Trideuteromethyl-3,5-dimethoxy-PEA |
| 51 | beta-D | 2.1.4 | Erowid | Lycaeum | beta,beta-Dideutero-3,4,5-trimethoxy-PEA |
| 52 | DESOXY | 2.1.2 | Erowid | Lycaeum | 4-Methyl-3,5-Dimethoxy-PEA |
| 53 | 2,4-DMA | 2.1.1 | Erowid | Lycaeum | 2,4-Dimethoxy-A |
| 54 | 2,5-DMA | 2.1.1 | Erowid | Lycaeum | 2,5-Dimethoxy-A |
| 55 | 3,4-DMA | 2.1.1 | Erowid | Lycaeum | 3,4-Dimethoxy-A |
| 56 | DMCPA | 2.2.2 | Erowid | Lycaeum | 2-(2,5-Dimethoxy-4-methylphenyl) -cyclopropylamine |
| 57 | DME | 2.1.1 | Erowid | Lycaeum | 3,4-Dimethoxy-beta-hydroxy-PEA |
| 58 | DMMDA | 2.3.3 | Erowid | Lycaeum | 2,5-Dimethoxy-3,4-methylenedioxy-A |
| 59 | DMMDA-2 | 2.3.3 | Erowid | Lycaeum | 2,3-Dimethoxy-4,5-methylenedioxy-A |
| 60 | DMPEA | 2.1.1 | Erowid | Lycaeum | 3,4-Dimethoxy-PEA |
| 61 | DOAM | 2.2.2 | Erowid | Lycaeum | 4-Amyl-2,5-dimethoxy-A |
| 62 | DOB | 2.2.2 | Erowid | Lycaeum | 4-Bromo-2,5-dimethoxy-A |
| 63 | DOBU | 2.2.2 | Erowid | Lycaeum | 4-Butyl-2,5-dimethoxy-A |
| 64 | DOC | 2.2.2 | Erowid | Lycaeum | 4-Chloro-2,5-dimethoxy-A |
| 65 | DOEF | 2.2.2 | Erowid | Lycaeum | 4-(2-Fluoroethyl)-2,5-dimethoxy-A |
| 66 | DOET | 2.2.2 | Erowid | Lycaeum | 4-Ethyl-2,5-dimethoxy-A |
| 67 | DOI | 2.2.2 | Erowid | Lycaeum | 4-Iodo-2,5-dimethoxy-A |
| 68 | DOM | 2.2.2 | Erowid | Lycaeum | 4-Methyl-2,5-dimethoxy-A |
| 69 | 2.2.2 | Erowid | Lycaeum | 4-Methyl-2,6-dimethoxy-A | |
| 70 | DON | 2.2.2 | Erowid | Lycaeum | 4-Nitro-2,5-dimethoxy-A |
| 71 | DOPR | 2.2.2 | Erowid | Lycaeum | 4-Propyl-2,5-dimethoxy-A |
| 72 | E | 2.1.3 | Erowid | Lycaeum | 4-Ethoxy-3,5-dimethoxy-PEA |
| 73 | EEE | 2.2.1 | Erowid | Lycaeum | 2,4,5-Triethoxy-A |
| 74 | EEM | 2.2.1 | Erowid | Lycaeum | 2,4-Diethoxy-5-methoxy-A |
| 75 | EME | 2.2.1 | Erowid | Lycaeum | 2,5-Diethoxy-4-methoxy-A |
| 76 | EMM | 2.2.1 | Erowid | Lycaeum | 2-Ethoxy-4,5-dimethoxy-A |
| 77 | ETHYL-J | 2.3.4 | Erowid | Lycaeum | N,a-diethyl-3,4-methylenedioxy-PEA |
| 78 | ETHYL-K | 2.3.4 | Erowid | Lycaeum | N-Ethyl-a-propyl-3,4-methylenedioxy-PEA |
| 79 | F-2 | 2.4.2 | Erowid | Lycaeum | Benzofuran-2-methyl-5-methoxy -6-(2-aminopropane) |
| 80 | F-22 | 2.4.2 | Erowid | Lycaeum | Benzofuran-2,2-dimethyl-5-methoxy -6-(2-aminopropane) |
| 81 | FLEA | 2.3.2 | Erowid | Lycaeum | N-Hydroxy-N-methyl-3,4-methylenedioxy-A |
| 82 | G-3 | 2.2.3 | Erowid | Lycaeum | 3,4-Trimethylene-2,5-dimethoxy-A |
| 83 | G-4 | 2.2.3 | Erowid | Lycaeum | 3,4-Tetramethylene-2,5-dimethoxy-A |
| 84 | G-5 | 2.2.3 | Erowid | Lycaeum | 3,4-Norbornyl-2,5-dimethoxy-A |
| 85 | GANESHA | 2.2.3 | Erowid | Lycaeum | 3,4-Dimethyl-2,5-dimethoxy-A |
| 86 | G-N | 2.2.3 | Erowid | Lycaeum | 1,4-Dimethoxynaphthyl-2-isopropylamine |
| 87 | HOT-2 | 2.2.2 | Erowid | Lycaeum | 2,5-Dimethoxy-N-hydroxy -4-ethylthio-PEA |
| 88 | HOT-7 | 2.2.2 | Erowid | Lycaeum | 2,5-Dimethoxy-N-hydroxy -4-(n)-propylthio-PEA |
| 89 | HOT-17 | 2.2.2 | Erowid | Lycaeum | 2,5-Dimethoxy-N-hydroxy -4-(s)-butylthio-PEA |
| 90 | IDNNA | 2.2.2 | Erowid | Lycaeum | 2,5-Dimethoxy-N,N-dimethyl-4-iodo-A |
| 91 | IM | 2.1.1 | Erowid | Lycaeum | 2,3,4-Trimethoxy-PEA |
| 92 | IP | 2.1.2 | Erowid | Lycaeum | 3,5-Dimethoxy-4-isopropoxy-PEA |
| 93 | IRIS | 2.2.3 | Erowid | Lycaeum | 5-Ethoxy-2-methoxy-4-methyl-A |
| 94 | J | 2.3.4 | Erowid | Lycaeum | a-Ethyl-3,4-methylenedioxy-PEA |
| 95 | LOPHOPHINE | 2.3.3 | Erowid | Lycaeum | 3-Methoxy-4,5-methylenedioxy-PEA |
| 96 | M (mescaline) | 2.1 | Erowid | Lycaeum | 3,4,5-Trimethoxy-PEA |
| 97 | 4-MA | 2.1.1 | Erowid | Lycaeum | 4-Methoxy-A |
| 98 | MADAM-6 | 2.3.2 | Erowid | Lycaeum | 2,N-Dimethyl-4,5-methylenedioxy-A |
| 99 | MAL | 2.1.2 | Erowid | Lycaeum | 3,5-Dimethoxy-4-methallyloxy-PEA |
| 100 | MDA | 2.3 | Erowid | Lycaeum | 3,4-Methylenedioxy-A |
| 101 | MDAL | 2.3.1 | Erowid | Lycaeum | N-Allyl-3,4-methylenedioxy-A |
| 102 | MDBU | 2.3.1 | Erowid | Lycaeum | N-Butyl-3,4-methylenedioxy-A |
| 103 | MDBZ | 2.3.1 | Erowid | Lycaeum | N-Benzyl-3,4-methylenedioxy-A |
| 104 | MDCPM | 2.3.1 | Erowid | Lycaeum | N-Cyclopropylmethyl-3,4-methylenedioxy-A |
| 105 | MDDM | 2.3.2 | Erowid | Lycaeum | N,N-Dimethyl-3,4-methylenedioxy-A |
| 106 | MDE | 2.3.1 | Erowid | Lycaeum | N-Ethyl-3,4-methylenedioxy-A |
| 107 | MDHOET | 2.3.1 | Erowid | Lycaeum | N-(2-Hydroxyethyl)-3,4-methylenedioxy-A |
| 108 | MDIP | 2.3.1 | Erowid | Lycaeum | N-Isopropyl-3,4-methylenedioxy-A |
| 109 | MDMA | 2.3.1 | Erowid | Lycaeum | N-Methyl-3,4-methylenedioxy-A |
| 110 | MDMC | 2.3.1 | Erowid | Lycaeum | N-Methyl-3,4-ethylenedioxy-A |
| 111 | MDMEO | 2.3.1 | Erowid | Lycaeum | N-Methoxy-3,4-methylenedioxy-A |
| 112 | MDMEOET | 2.3.1 | Erowid | Lycaeum | N-(2-Methoxyethyl)-3,4-methylenedioxy-A |
| 113 | MDMP | 2.3.2 | Erowid | Lycaeum | a,a,N-Trimethyl-3,4-methylenedioxy-PEA |
| 114 | MDOH | 2.3.1 | Erowid | Lycaeum | N-Hydroxy-3,4-methylenedioxy-A |
| 115 | MDPEA | 2.3.1 | Erowid | Lycaeum | 3,4-Methylenedioxy-PEA |
| 116 | MDPH | 2.3.2 | Erowid | Lycaeum | a,a-Dimethyl-3,4-methylenedioxy-PEA |
| 117 | MDPL | 2.3.1 | Erowid | Lycaeum | N-Propargyl-3,4-methylenedioxy-A |
| 118 | MDPR | 2.3.1 | Erowid | Lycaeum | N-Propyl-3,4-methylenedioxy-A |
| 119 | ME | 2.1.3 | Erowid | Lycaeum | 3,4-Dimethoxy-5-ethoxy-PEA |
| 120 | MEDA | 2.3.3 | Erowid | Lycaeum | 3,4-Ethylenedioxy-5-methoxy-A |
| 121 | MEE | 2.2.1 | Erowid | Lycaeum | 2-Methoxy-4,5-diethoxy-A |
| 122 | MEM | 2.2.1 | Erowid | Lycaeum | 2,5-Dimethoxy-4-ethoxy-A |
| 123 | MEPEA | 2.1.1 | Erowid | Lycaeum | 3-Methoxy-4-ethoxy-PEA |
| 124 | META-DOB | 2.2.2 | Erowid | Lycaeum | 5-Bromo-2,4-dimethoxy-A |
| 125 | META-DOT | 2.2.2 | Erowid | Lycaeum | 5-Methylthio-2,4-dimethoxy-A |
| 126 | METHYL-DMA | 2.1.1 | Erowid | Lycaeum | N-Methyl-2,5-dimethoxy-A |
| 127 | METHYL-DOB | 2.2.2 | Erowid | Lycaeum | 4-Bromo-2,5-dimethoxy-N-methyl-A |
| 128 | METHYL-J | 2.3.4 | Erowid | Lycaeum | N-Methyl-a-ethyl-3,4-methylenedioxy-PEA |
| 129 | METHYL-K | 2.3.4 | Erowid | Lycaeum | N-Methyl-a-propyl-3,4-methylenedioxy-PEA |
| 130 | METHYL-MA | 2.1.1 | Erowid | Lycaeum | N-Methyl-4-methoxy-A |
| 131 | METHYL-MMDA-2 | 2.3.3 | Erowid | Lycaeum | N-Methyl-2-methoxy-4,5-methylenedioxy-A |
| 132 | MMDA | 2.3.3 | Erowid | Lycaeum | 3-Methoxy-4,5-methylenedioxy-A |
| 133 | MMDA-2 | 2.3.3 | Erowid | Lycaeum | 2-Methoxy-4,5-methylenedioxy-A |
| 134 | MMDA-3a | 2.3.3 | Erowid | Lycaeum | 2-Methoxy-3,4-methylenedioxy-A |
| 135 | MMDA-3b | 2.3.3 | Erowid | Lycaeum | 4-Methoxy-2,3-methylenedioxy-A |
| 136 | MME | 2.2.1 | Erowid | Lycaeum | 2,4-Dimethoxy-5-ethoxy-A |
| 137 | MP | 2.1.2 | Erowid | Lycaeum | 3,4-Dimethoxy-5-propoxy-PEA |
| 138 | MPM | 2.2.1 | Erowid | Lycaeum | 2,5-Dimethoxy-4-propoxy-A |
| 139 | ORTHO-DOT | 2.2.2 | Erowid | Lycaeum | 2-Methylthio-4,5-dimethoxy-A |
| 140 | P | 2.1.2 | Erowid | Lycaeum | 3,5-Dimethoxy-4-propoxy-PEA |
| 141 | PE | 2.1.2 | Erowid | Lycaeum | 3,5-Dimethoxy-4-phenethyloxy-PEA |
| 142 | PEA | 1.2 | Erowid | Lycaeum | PEA |
| 143 | PROPYNYL | 2.1.2 | Erowid | Lycaeum | 4-Propynyloxy-3,5-dimethoxy-PEA |
| 144 | SB | 2.1.3 | Erowid | Lycaeum | 3,5-Diethoxy-4-methoxy-PEA |
| 145 | TA | 2.1.1 | Erowid | Lycaeum | 2,3,4,5-Tetramethoxy-A |
| 146 | 3-TASB | 2.1.3 | Erowid | Lycaeum | 4-Ethoxy-3-ethylthio-5-methoxy-PEA |
| 147 | 4-TASB | 2.1.3 | Erowid | Lycaeum | 3-Ethoxy-4-ethylthio-5-methoxy-PEA |
| 148 | 5-TASB | 2.1.3 | Erowid | Lycaeum | 3,4-Diethoxy-5-methylthio-PEA |
| 149 | TB | 2.1.2 | Erowid | Lycaeum | 4-Butylthio |
| 150 | 3-TE | 2.1.2 | Erowid | Lycaeum | 4-Ethoxy-5-methoxy-3-methylthio-PEA |
| 151 | 4-TE | 2.1.2 | Erowid | Lycaeum | 3,5-Dimethoxy-4-ethylthio-PEA |
| 152 | 2-TIM | 2.1.4 | Erowid | Lycaeum | 2-Methylthio-3,4-dimethoxy-PEA |
| 153 | 3-TIM | 2.1.4 | Erowid | Lycaeum | 3-Methylthio-2,4-dimethoxy-PEA |
| 154 | 4-TIM | 2.1.4 | Erowid | Lycaeum | 4-Methylthio-2,3-dimethoxy-PEA |
| 155 | 3-TM | 2.1.4 | Erowid | Lycaeum | 3-Methylthio-4,5-dimethoxy-PEA |
| 156 | 4-TM | 2.1.4 | Erowid | Lycaeum | 4-Methylthio-3,5-dimethoxy-PEA |
| 157 | TMA | 2.1.1 | Erowid | Lycaeum | 3,4,5-Trimethoxy-A |
| 158 | TMA-2 | 2.1.1 | Erowid | Lycaeum | 2,4,5-Trimethoxy-A |
| 159 | TMA-3 | 2.1.1 | Erowid | Lycaeum | 2,3,4-Trimethoxy-A |
| 160 | TMA-4 | 2.1.1 | Erowid | Lycaeum | 2,3,5-Trimethoxy-A |
| 161 | TMA-5 | 2.1.1 | Erowid | Lycaeum | 2,3,6-Trimethoxy-A |
| 162 | TMA-6 | 2.1.1 | Erowid | Lycaeum | 2,4,6-Trimethoxy-A |
| 163 | 3-TME | 2.1.2 | Erowid | Lycaeum | 4,5-Dimethoxy-3-ethylthio-PEA |
| 164 | 4-TME | 2.1.2 | Erowid | Lycaeum | 3-Ethoxy-5-methoxy-4-methylthio-PEA |
| 165 | 5-TME | 2.1.2 | Erowid | Lycaeum | 3-Ethoxy-4-methoxy-5-methylthio-PEA |
| 166 | 2T-MMDA-3a | 2.3.3 | Erowid | Lycaeum | 2-Methylthio-3,4-methylenedioxy-A |
| 167 | 4T-MMDA-2 | 2.3.3 | Erowid | Lycaeum | 4,5-Thiomethyleneoxy-2-methoxy-A |
| 168 | TMPEA | 2.1.1 | Erowid | Lycaeum | 2,4,5-Trimethoxy-PEA |
| 169 | 2-TOET | 2.2.2 | Erowid | Lycaeum | 4-Ethyl-5-methoxy-2-methylthio-A |
| 170 | 5-TOET | 2.2.2 | Erowid | Lycaeum | 4-Ethyl-2-methoxy-5-methylthio-A |
| 171 | 2-TOM | 2.2.2 | Erowid | Lycaeum | 5-Methoxy-4-methyl-2-methylthio-A |
| 172 | 5-TOM | 2.2.2 | Erowid | Lycaeum | 2-Methoxy-4-methyl-5-methylthio-A |
| 173 | TOMSO | 2.2.2 | Erowid | Lycaeum | 2-Methoxy-4-methyl-5-methylsulfinyl-A |
| 174 | TP | 2.1.2 | Erowid | Lycaeum | 4-Propylthio-3,5-dimethoxy-PEA |
| 175 | TRIS | 2.1.2 | Erowid | Lycaeum | 3,4,5-Triethoxy-PEA |
| 176 | 3-TSB | 2.1.2 | Erowid | Lycaeum | 3-Ethoxy-5-ethylthio-4-methoxy-PEA |
| 177 | 4-TSB | 2.1.2 | Erowid | Lycaeum | 3,5-Diethoxy-4-methylthio-PEA |
| 178 | 3-T-TRIS | 2.1.2 | Erowid | Lycaeum | 4,5-Diethoxy-3-ethylthio-PEA |
| 179 | 4-T-TRIS | 2.1.2 | Erowid | Lycaeum | 3,5-Diethoxy-4-ethylthio-PEA |