It is known that lysergic acid can be prepared by isomerizing paspalic acid, using potassium hydroxide2 or sodium hydroxide3, but these processes do not allow either good yields or a product comprising small quantities of isolysergic acid to be obtained industrially. A process has now been found, allowing lysergic acid of sufficient purity to be obtained in very good yields by isomerizing paspalic acid using a tetraalkylammonium hydroxide1, preferably tetrabutylammonium hydroxide. It is also possible to use the tetraalkylammonium hydroxide in a mixture with a small quantity of an alkali metal hydroxide, preferably sodium hydroxide.
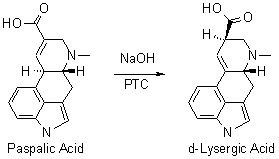
The process is generally carried out in an inert solvent, such as water or a short-chain aliphatic alcohol (methanol or ethanol, for example), or in a mixture of these solvents, at a temperature preferably between 25-35°C, and then letting the reaction progress for 24 hours. The quantity of tetraalkylammonium hydroxide is generally from 1.5-10 mol and preferably 2.5 mol per mole of paspalic acid. When use is made of a mixture of tetraalkylammonium hydroxide and alkali metal hydroxide, the quantity of tetraalkylammonium hydroxide is generally from 0.5-2 mol, preferably 1.5 mol, and the quantity of alkali metal hydroxide is generally from 4.5-0.5 mol, preferably 1 mol, per mole of paspalic acid. Still more preferably, the process is carried out in an aqueous medium at a temperature of from 28-32°C., either in the presence of 2.5 mol of tetrabutylammonium hydroxide for one mole of paspalic acid or in the presence of 1.5 mol of tetrabutylammonium hydroxide and of 1 mol of sodium hydroxide for one mole of paspalic acid.
The lysergic acid is then precipitated by acidifying the reaction medium, using a mineral acid, preferably using sulphuric acid, and is filtered. It is advantageous to acidify to a pH of about 3-4, in particular to 3.5, while not exceeding 30°C.
Experimental
Example 11
130 g of paspalic acid are added rapidly to 780.1 g of a 40% strength solution of tetrabutylammonium hydroxide in water, with stirring and under a stream of nitrogen. The mixture is brought to 30±2°C. and allowed to react at this temperature for 20 hours. The reaction medium is cooled to about 20°C and held at this temperature for 3.5 h 918 g of water are added, followed by acidification using 95% strength sulphuric acid until the pH is 3.5, while maintaining the temperature at about 30°C. The reaction mixture is then cooled to 10±2°C. and held at this temperature for 30 minutes. The mixture is filtered through a sinter funnel under a vacuum of 0.4 bar, washed with 3x300 ml of water and then dried for 14 h at 75±2°C and 20 mbar. This gives 107 g of lysergic acid comprising less than 3% of isolysergic acid and the yield (weight/weight) is 80%.
Example 21
130 g of paspalic acid are added rapidly to 472.3 g of a 40% strength solution of tetrabutylammonium hydroxide in water, with stirring and under a stream of nitrogen, followed by 316 g of a 1.5N aqueous solution of sodium hydroxide. The mixture is brought to 30±2°C. and allowed to react at this temperature for 20 hours. The reaction medium is cooled to about 20°C. and held at this temperature for 3 h 30 min. 918 g of water are added, followed by acidification using 95% strength sulphuric acid until the pH is 3.5, while maintaining the temperature at about 30°C. The reaction mixture is then cooled to 10±2°C. and held at this temperature for 30 minutes. The mixture is filtered through a sinter funnel under a vacuum of 0.4 bar, washed with 3x300 ml of water and then dried for 14 hours at 75±2°C. and 20 mbar. This gives 107.8 g of lysergic acid comprising only 2.8% of isolysergic acid and the yield (w/w) is 81.6%.
Preparation of Lysergic Acid with Sodium Hydroxide Alone3
5g of paspalic acid in 100 ml of a 2N aqueous solution of sodium hydroxide are heated at reflux for 2 hours. After cooling, the pH of the reaction medium is brought to 5.5 by adding an aqueous solution of hydrochloric acid and glacial acetic acid (20 ml of water, 10 ml of HCl and 10 ml of acetic acid). The precipitate is filtered, washed with 3x20 ml of 50% aqueous methanol, then dried in vacuo at 75°C. This gives 3.15 g of lysergic acid comprising 6.8% of isolysergic acid and the yield (w/w) is 59.3%.
Preparation of Lysergic Acid with Potassium Hydroxide Alone2
5g of paspalic acid in 360 g of a 0.5N solution of KOH in a 50% aqueous ethanol is heated at reflux for 1 hour. After cooling, the pH of the reaction medium is brought to 5.5 by adding 1N HCl. The precipitate is filtered, washed with 3x20 ml 50% aqueous methanol, then dried in vacuo at 75°C. This gives 2.86 g of lysergic acid comprising 1% of isolysergic acid and the yield (w/w) is 49.8%.