Synthesis of Phenethylamines from Phenylacetic Acids[ Back to the Chemistry Archive ] This is a general method for making Phenetylamines from the corresponding Phenylacetic acids, which in turn can be synthesized in many ways. 3,4,5-trimethyl-phenethylamine itself seems to be an active compound, but it is not as of yet tested in humans. Here is an excerpt from Pihkal: "3,4,5-Trimethylphenethylamine have been studied in the cat, and have shown extraordinary pharmacological profiles of CNS action. The trimethyl compound showed behavior that was interpreted as being intense mental turmoil, accompanied by a startling rise in body temperature. The significance is hard to determine, in that LSD gave similar responses in the cat, but mescaline was without effects at all. No human studies have been made on these compounds, just animal studies. But they might prove upon trial in man to be most revealing. They would have to be performed with exceptional care." 3,4,5-Trimethylphenylacetamide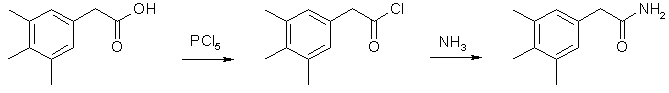
21.3 g (0.12 mol) of 3,4,5-trimethylphenylacetic acid and 25g (0.12 mol) PCl5 was mixed in a RB flask with a reflux condenser, and after the initial vigorous reaction had subsided, the mixture was warmed on the steam bath for 10 min. The mixture was distilled under reduced pressure to remove phosphorous oxychloride, and the residue was added gradually to 100 ml of ice-cooled 25% aqueous ammonia. The precipitated amide was collected, washed with water, and air dried; recrystallization from benzene plus a small amount of ethanol afforded 18 g (85%) of the pure amide, mp 183-184°C. 3,4,5-Trimethyl-phenethylamine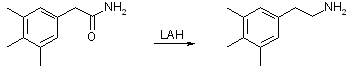
To a stirred suspension of 8.6g (226 mmol) of lithium aluminum hydride in 500 ml of absolute ether, was added a solution of 10g (56 mmol) of 3,4,5-trimethylphenylacetamide in 600 ml of boiling reagent benzene, using additional hot benzene to redissolve material which crystallized during the addition. The reaction mixture was stirred and refluxed for 22 h and then hydrolyzed by cautious addition of water and 10% sulfuric acid. A white solid insoluble in both the ether and aqueous layers was formed and collected by filtration. This material proved to be the insoluble sulfate of 3,4,5-trimethyl-phenethylamine contaminated by aluminum salts. Upon heating with concentrated hydrochloric acid, the crude product dissolved, and the hydrochloride of 3,4,5-trimethyl-phenethylamine crystallized in the form of colorless lustrous plates on cooling. The yield was 10.1 g (89%), mp 249-250°C after recrystallization form methanol-ethyl acetate. References[1] JOC 22, 332-333 (1957) |