Introduction
Phenyl-2-Propanone (Phenyacetone, P2P) can be made in a single step by a free-radical reaction between benzene and acetone1,2. The reaction relies upon the special oxidative powers of manganese(III) acetate, Mn(OAc)3. Normally when a compound is oxidized, two electrons are removed from the compound (forming a charged ion), but manganese(III) acetate is able to remove only one, creating a very reactive free radical, which reacts with almost anything nearby.
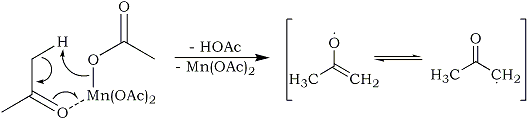
In this case, the manganese(III) acetate reacts with acetone to form an acetone radical, acetic acid and manganese(II) acetate:
The formed acetone radical immediately reacts with a benzene molecule, forming a non-aromatic intermediate radical, which with the aid of a second molecule of manganese(III) acetate is oxidatively deprotonated to form phenyl-2-propanone:
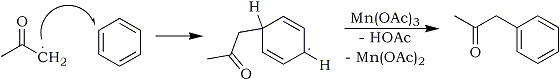
As you can see, the overall reaction is that two manganese(III) acetate molecules aids the coupling of benzene and acetone, forming two acetic acid molecules in the process, while it itself is reduced to manganese(II) acetate. The manganese(II) acetate ends up in the aqueous washes of the post-reaction mixture, and the manganese(II) can be reclaimed by precipitating it as the insoluble carbonate by addition of a concentrated sodium carbonate solution, and the carbonate can be recycled into pure manganese(II)acetate again as detailed in the manganese(III) acetate document at this site.
There is also a possibility that the manganese(II) acetate can be regenerated to manganese(III) acetate in situ in the reaction mixture, either by slowly adding KMnO4 to the reaction mixture (used in the manganese(III) acetate-mediated acetonylation of cis-olefins3), or by electrochemical oxidation directly in the reaction mixture (used in the manganese(III) acetate-mediated nitromethylation of benzene4). However, the precise reaction conditions for these procedures in the acetonylation of benzene remains undetermined, but in the references mentioned they have found the procedures to work satisfactorily, and there seems to be no obvious reasons why it would not also work in this particular reaction. If a working manganese(III) acetate regeneration procedure is developed, this reaction should be one of the easiest, cheapest and least suspicious procedures for making phenyl-2-propanone to date.
As the formed acetone radicals are so reactive, the reaction has to be performed in a pretty dilute solution, or else the acetone radicals may combine with themselves to form dimers (with methyl ethyl ketone, almost only such dimers formed, and no addition to the benzene ring). The formed acetone radicals does not add too selectively to aromatic rings, with benzene there is only one possible product, but in the case of mono- substituted benzenes, the products were all three possible isomers, when anisole (methoxybenzene) was subjected to this treatment, the product distribution was o-methoxyphenyl-2-propanone (84.3%), m-methoxy- phenyl-2-propanone (2.6%) and p-methoxyphenyl-2-propanone (13.1%), with a 75% total yield of phenyl-2- propanones. With symmetrical disubstituted benzenes only one product is obtained, as in the preparation of 2,5-dimethoxyphenyl-2-propanone from 1,4-dimethoxybenzene (This compound is a very useful starting material for the synthesis of DOB and related psychedelic amphetamines). If alkylbenzenes such as toluene is used, benzyl acetate is produced as a by-product (in about 10-15% yield), because of the reactive nature of the benzylic carbon.
There is a possibility that the dilution of reagents used can be optimized further than the amounts described in the example below, but it is unknown how far the reduction of reagents can be reduced without side reactions occurring. In the original reference of this reaction1, 0.1 mole of manganese(III) acetate dihydrate (26.8g) was reacted with 1 mole of acetone (58g) and 0.5 mole of benzene (39g) in 100ml glacial acetic acid at 70°C to give 36% Phenyl-2-propanone, based on reacted manganese(III) acetate. As this almost five-fold increase in concentration only gave a 4% reduction in yield, there is reason to believe that the very large excesses of reactants are necessary for a successful reaction.
Experimental2
A mixture of Manganese(III) acetate dihydrate (13.4g, 50 mmol), benzene (150ml), acetone (150ml) and glacial acetic acid (250ml) was refluxed under an inert atmosphere (argon, helium or nitrogen) until the dark brown color of manganese(III) acetate changed to the pale pink of manganese(II) acetate (about 90 min). The reaction mixture was partitioned between 400ml ether and 250ml water. The ether layer was separated and washed with 250ml water and with 2x250ml 5% Na2CO3 to remove any remaining acetic acid. The ether was then dried over anhydrous Na2SO4 (or MgSO4), the solvent evaporated and the residue fractionally distilled to recover unreacted benzene, and to give phenyl-2-propanone in 40% yield (1.34g) based on the reacted manganese(III) acetate, which is the limiting reagent in this reaction.
If anhydrous Manganese(III) acetate is used, 50 mmol corresponds to 10.1g instead of the 13.4g of the dihydrate. When solid aromatics was used as substrates, the amounts used were reduced to facilitate the work-up process. In the case of 1,4-dimethoxybenzene, 100g (720 mmol) was used instead. Of course any unreacted starting material is recovered by distillation after the reaction and reused in another run.
The variations in reaction times for different substrates, as well as the yields (with product distribution in the case of monosubstituted aromatics) are presented in the table below.
| Substrate | P2P Total Yield |
Rxn Time (min) |
Product Distribution |
||||
| Benzene |
40% |
90 |
Phenyl-2-Propanone (100%) | ||||
| Toluene |
51% |
60 |
o-Me-P2P (66%) | m-Me-P2P (20%) | p-Me-P2P (15%) | ||
| Anisole |
75% |
45 |
o-MeO-P2P (84.3%) | m-MeO-P2P (2.6%) | p-MeO-P2P (13.1%) | ||
| Chlorobenzene |
25% |
- |
o-Cl-P2P (72.1%) | m-Cl-P2P (6%) | p-Cl-P2P (21.9%) | ||
| Fluorobenzene |
29% |
105 |
o-F-P2P (70.10%) | m-F-P2P (9.6%) | p-F-P2P (19.10%) | ||
| Naphtalene |
71% |
25 |
α-Naphtyl-2-propanone (92.1%), β-Naphtyl-2-propanone (7.9%) |
||||
| 1,4-MeO-benzene |
39% |
40 |
2,5-Dimethoxy-Phenyl-2-Propanone (100%) | ||||