The Étard reaction (the reaction of hydrocarbons with chromyl chloride) has been studied for many years2. In spite of this, little is known about the reactions of compounds other than toluene. The problems associated with this reaction are illustrated by the case of n-propylbenzene which gives propiophenone and benzyl methyl ketone as the products3. The latter is the major product, although it involves oxidation at a site one removed from the reactive benzylic position. The complex itself is an amorphous, hygroscopic solid which resists efforts at determining its structure directly.
It may be established that the reaction proceeds first at the benzylic position. The oxidation of t-butylbenzene proceeds at a very slow rate compared to compounds having an available α-hydrogen4. Similarly, in aliphatic cases, cyclohexane reacts at a much slower rate than does methylcyclohexane. From this it appears that the major product of the oxidation of n-propylbenzene, benzyl methyl ketone, must arise from a rearrangement. In order to confirm this, n-propyl-β-d2-benzene was prepared by the equilibration of propiophenone with deuterium oxide, followed by reduction using lithium aluminum hydride and aluminum chloride5. Oxidation led to benzyl methyl ketone having 60% of one deuterium in the α-position. Thus, hydrogen migration occurs in the formation of the β-oxidation product.
Two other significant observations may be made concerning the reaction. First, the yield of ketonic material obtained on the hydrolysis of the Étard complex is less than 50%. Much of the rest of the organic material is found in solvent used in the formation of the complex (carbon tetrachloride), and it is largely in the form of α-chloropropylbenzene. Thus, chlorination (using chlorine free chromyl chloride) is an important side reaction.
Table 1
Effect of Reactant Ratio on Product Distribution*
Reactants | Products | |||||
Chromyl Chloride | Propyl- benzene | Propyl- benzene | α-Chloro- propyl- benzene | Benzyl Methyl Ketone | Propio- phenone |
Ketone Ratio |
8.0 g | 6.0 | 1.63 | 1.38 | 0.31 | - |
5.7 |
- | 0.32 | 0.56 | 0.15 | |||
16.0 | 6.0 | 0.05 | 1.60 | 0.96 | - |
4.1 |
- | 0.32 | 0.78 | 0.42 | |||
24.0 | 6.0 | - | 1.64 | 0.40 | - |
2.1 |
- | 0.23 | 0.52 | 0.45 | |||
32.0 | 6.0 | - | 0.66 | 0.11 | - | 0.7 |
- | 0.28 | 0.61 | 0.94 |
|||
* Under products, the upper line in each case represents
the material found in the solvent, and the lower (dark grey) line
gives
the products isolated from the hydrolysis of the complex.
Second, the product composition depends on the concentration of chromyl chloride. This may be seen in the data in Table 1. With a deficiency of chromyl chloride, the benzyl methyl ketone: propiophenone ratio is 5.7 and it decreases to less than unity when an excess of chromyl chloride is used. This is not due to preferential decomposition of the benzyl methyl ketone precursor by chromyl chloride since the amount of propiophenone increases with increasing chromyl chloride concentration. These data show that the initial reaction occurs at the α-position leading either to ketonic or halogen containing compounds, and that the two ketones are formed by two different paths after the initial step. The data suggest that the second step in the formation of benzyl methyl ketone does not involve chromyl chloride, and that the corresponding step in the formation of propiophenone does.
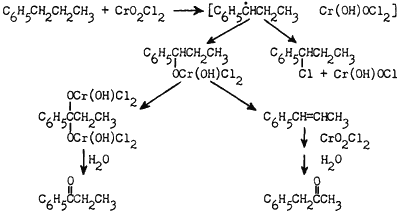
A scheme which is in accord with these data and with our previous work on the chromic acid oxidation of hydrocarbons6 can be seen in Figure 1.
Alternately, the initial step may be written as a free radical chain process. If a free radical were formed initially, and reacted either in the solvent cage or with a molecule of chromyl chloride, it would be reasonable to expect reaction at either oxygen or chlorine leading to the two types of observed products.
When the concentration of chromyl chloride is low, the initial intermediate may eliminate Cr(IV) giving the alkene. We have found that β-methylstyrene gives exclusively benzyl methyl ketone in the Étard reaction. If the chromyl chloride concentration is high, the bimolecular reaction of the intermediate becomes favored giving a normal Étard complex of the type formed from toluene. Hydrolysis then gives propiophenone.
This mechanistic hypothesis appears to fit all of the data on compounds which undergo the Étard reaction, except for methylcyclohexane which has been reported to give hexahydrobenzaldehyde7. We have repeated this oxidation and have been unable to find hexahydrobenzaldehyde. The products are 2-methylcyclohexanone and 2-methyl-2-chlorocyclohexanone. These are the expected products based on the above mechanism.