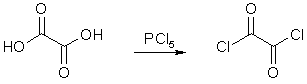
126g (1 mole) of anhydrous oxalic acid is ground into a fine powder and mixed slowly and thoroughly with 400g (1.92 moles) of powdered phosphorous pentachloride (PCl5) with efficient cooling in an ice bath. Chlorine gas is evolved, so it is important to perform the reaction under an efficient fume hood. The mixture was allowed to return to room temperature slowly, and was left standing for 48-72h until the mixture liquifies completely. The mixture is now distilled, collecting the fraction boiling between 60-100°C. Repeat the distillation until the phosphorus-free, pure oxalyl chloride, bp 63-64°C, is obtained. Yield approximately 45-50% of theory. Fractional distillation would most likely allow a quicker separation but the author made no mention of this.