Abstract
The reduction of 1-nitro-1-alkenes with leadľacetic acid in dimethylformamide afforded the corresponding aldoximes (5 examples) or ketoximes (3 examples) in excellent yield.
The chemistry of nitroalkenes has been extensively investigated and their synthetic utility is well documented.1 Thus, nitroalkenes have been converted to such synthetically useful compounds2 as carbonyl compounds by low valent metal species,3,4 Raney Ni-NaH2PO4,5LiBH(sec-Bu)3.6 and Bu3SnH-m-chloroperbenzoic acid or O3;7 alkenamines by catalytic hydrogenolysis;8 substituted hydroxylamines by BH3;9 substituted oximes by SnCl2;10 pyrroles by TiCl3;11 and nitriles by electroreduction in the presence of TiCl4.12 However, most of the current preparative methods of oximes from nitroalkenes are not versatile. Reduction of nitroalkenes by CrCl213 or NaH2PO2 in the presence of palladium14 was reported to afford the corresponding oximes, but the yield was not satisfactory. Zn-acetic acid15and Na2SnO216 reductions are limited to the preparation of ketoximes only. Electroreduction of nitroalkenes was reported to yield mixtures of ketones and ketoximes,17 or oximes and acetals (or ketones)18 depending on the structure of nitroalkenes.
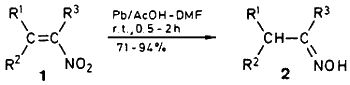
The present communication concerns the reduction of nitroalkenes 1 by means of lead powder in an acetic acid-DMF solvent to give the corresponding oximes 2 in high yields. The procedure is simple and the oxime is obtained as a sole product.
Table
Reduction of Nitroalkenes (1) to Oximes (2)
Nitroalkene | R1 |
R2 |
R3 |
Reaction time |
Yielda |
E:Z-Ratiob |
1a |
Ph | H | H | 30 min | 94% | 1:1-3:1 |
1b | 3,4-(MeO)2Ph |
H | H | 2 h | 85% | 5:4 |
1c |
1-Naphthyl | H | H | 40 min | 85% | 7:2 |
1d | C6H13 |
H | H | 2 h | 87% | 1:1-3:1 |
1e |
Ph | Ph | H | 2 h | 71% | 3:2 |
1f |
Ph | H | Me | 2 h | 92% | 6:1 |
1g |
Ph | H | Ph | 2 h | 93% | - |
1h |
Ph | Ph | Me | 2 h | 88% | 1:1 |
a Isolated yields.
b Determined by 1H-NMR.
During our investigation on electroreduction of nitroalkenes,12 we found that metallic lead (plates or powder) can reduce nitroalkenes 1 to give oximes 2 in an acetic acid-DMF solution without electricity. Reduction of 1 with metallic lead has not been reported. Torii and his co-workers17 have reported that the electrochemically beforehand activated lead plate (the electrode) reduced 1-(4-chlorophenyl)- 2-nitropropene to give a mixture of 1-(4-chlorophenyl)- 2-propanone and its oxime (total 90%, in aq. perchloric acid-CH2Cl2), but without electroactivation most of the starting nitroalkene was recovered unchanged. The present lead reduction of 1 in acetic acid-DMF (1:25, v/v) does not need the electroactivation at all. As shown in the Table, both the ketoximes and aldoximes are obtained in excellent yields after usual chromatographic separation.
The present reduction provides a simple and versatile preparative method of oximes from nitroalkenes which can be easily prepared by condensation of carbonyl compounds with nitroalkanes.
Standard Procedure
A nitroalkene (1, 0.01 mol) was dissolved in a mixture of acetic acid (5 mL) and DMF (75 mL), and lead powder (2 equivalents) was added. The mixture was stirred at ambient temperature for a period given in the Table. The mixture was then poured into ice-water, and extracted with diethyl ether. The ether solution was washed successively with an aq. NaHCO3 solution and an aq. NaCl solution, and then dried over MgSO4. The solvent was evaporated off and the resultant crude oxime 2 was purified by chromatography (silica gel/CH2Cl2).