Synthesis of Moclobemideby Psycho ChemistIntroductionMoclobemide = N-(2-morpholinoethyl)-4-chlorobenzamide
I suffered severe depression, and doctors refused to prescribe moclobemide to me at first, so I looked up the patent and made me some. The dose is 150 mg in the morning and 300 mg after lunch. Never use moclobemide with amphetamines, phenethylamines or the like, as severe blood pressure rush, heart attacks and even death may be the result! (From a pharmaceutical book). Moclobemide is totally legal here, and not controlled. Watched chemicals are not necessary. I don't know the legality of Moclobemide in the US. The procedure is not dangerous, but only people with chemical knowledge should carry out the synthesis. N-(2-aminoethyl)-morpholineTo 1875 g 57,5% ethylenediamine in water (18 moles) in a 5 L flask equipped with agitator, reflux condenser and dropping funnel, 863 g (6 moles) of bis(2-chloroethyl)-ether was added during one hour. Thirty minutes later, 505 g sodium hydroxide was added cautiously. The mixture was cooled and the precipated salt filtered. The liquor was dried over sodium hydroxide (*) and then distilled through a 600x50mm packed column. After distillation of excess ethylenediamine (recycle!), 370 g (47.5 % of theoretical) of N-(2-aminoethyl)-morpholine boiling at 121-123°C (68 mm) , b.p. 204.5°C (768 mm), was obtained as a water-white liquid with a refractive index of 1.4742 (25 °C). These values were in agreement with those of N-(2-aminoethyl)-morpholine preparated by the phthalimide process. * A semisolid or oily bottom layer of NaOH will form (300-400 g of NaOH should be added), which is separated. Maybe the use of 18 moles of 99 % ethylenediamine is better, the workup would be easier. Bis(2-chloroethyl)-ether is a carcinogen and acute toxic chemical. Moclobemide = N-(2-morpholinoethyl)-4-chlorobenzamide26 g (0.2 moles) N-(2-aminoethyl)-morpholine is dissolved in 200 mL pyridine (dry), 35 g (0.2 moles) 4-chlorobenzoyl chloride is added dropwise with stirring and ice-cooling (addition time = 2 hrs). After stirring at room temperature overnight, the pyridine is evaporated using a rotavap (recycle!), and twice 200 mL toluene are added to the residue and evaporated in vacuum (rotavap) from the residue to remove pyridine rests. (The distilled toluene is thereafter acid-washed, dried and redistilled for reuse.) The residue is dissolved with shaking in a mixture of 300 mL water and 300 mL dichloromethane, and 3M potassium hydroxide solution is added until pH is 10, and the lower organic layer is separated. The aqueous layer is once again extracted with 100 mL dichloromethane. The combined dichloromethane extracts are dried with 20 g sodium sulfate, which is then filtered off and washed with 50 mL dichloromethane and evaporated (rotavap). The residue is recrystallized from 180 mL 2-propanol/water 9:1 (volume/volume), a second crop is obtained by evaporating the mother liquor to 75 mL. Thus 40-45g (74-84% total yield) of moclobemide is obtained. Store in a dark and clean bottle. 200 mL pyridine can easily be substituted with 350 mL triethylamine (dry). The pyridine or triethylamine are distilled from sodium metal prior to use, and the N-(2-Aminoethyl)-morpholine and 4-chlorobenzoyl chloride are freshly vacuum distilled. 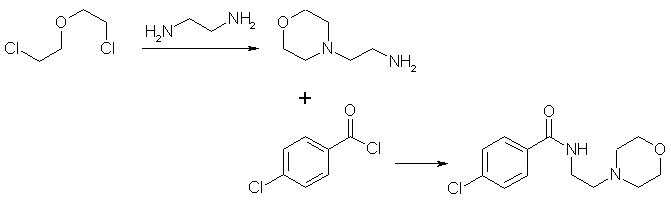 Moclobemide synthesis literatureRegistry Numbers71320-77-9 (free base) 64544-22-5 (hydrochloride) 108375-13-9 (hydrochloride monohydrate) References Journal of the American Chemical Society, Vol.62, page 447, 1940 N-(2-aminoethyl)-morpholine Acta Pol. Pharm. Vol. 30, page 135 - 143, year 1973 Chem. Abstr. 79/105217kUnchlorinated moclo analog, "parent structure" German patent 2,706,179 (Chem Abs 88, 121196u) Austrian patent 349,480 (Chem Abs 91, 123739j) Austrian patent 349,481 (Chem Abs 91, 123740c) Austrian patent 349,482 (Chem Abs 91, 123741d) Austrian patent 349,479 (Chem Abs 91, 123742e) US-patent 4,210,754 (Chem Abs 94, 65702r) All these patents contain the same preparation methods. The German and the US-patent are most easy to get. J. Med. Chem. 36, 3968-3970, year 1993 (Chem Abs 120, 106492y) Describes MAO-B inhibitors, moclobemide analogs Pharm. Acta Helv. 72, 119-122, (1997) (Chem Abs 126, 301399z) Piperidine and pyrrolidine analog of moclobemide described Moclobemide analog Befol (N-(3-morpholinopropyl)-4-chlorobenzamide) This is the russian moclobemide analog. Chem Abs 110, 8221h Chem Abs 112, 131889a Chem Abs 114, 42692e Chem Abs 114, 49709z |