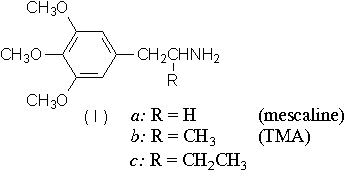
The establishment of trimethoxyamphetamine (Ib, TMA) as being of greater psychotropic potency than either a-ethyl mescaline (Ic)1 or mescaline itself (Ia)2 has led to the examination of analogues which retain the three carbon chain but vary in other areas of the molecule. The presence of elemicin (3,4,5-trimethoxy allylbenzene) in the aromatic ether fraction of oil of nutmeg3, and the proposal of a possible in vivo mechanism of its conversion to TMA4 have afforded an explanation of the psychotropic action5 of nutmeg. The substance myristicin (II) is the major component of this fraction, and if it were to undergo the transformation parallel to that proposed for elemicin, a second amphetamine, 3-methoxy-4,5-methylenedioxy amphetamine (MMDA, IVa), would result.
This latter amphetamine has been synthesized from myristicin in vitro, by a three-step process. trans-Isomyristicin, obtained from myristicin by heating in alcoholic potassium hydroxide, is converted by means of tetranitromethane to ß-nitro isomyristicin. Reduction of this nitropropene with LiAlH4 led smoothly to MMDA, which was isolated as the hydrochloride. Examination of MMDA in mice and dogs displayed a behavioural and toxicological pattern similar to that of TMA, both quantitatively and qualitatively. Preliminary human evaluation revealed initial intoxication at about 1.0 mg/kg (as free base). More extensive assay has confirmed a thoroughly effective hypnogogic dementia at less than 2.0 mg/kg. The psychotomimetic syndrome was complete at this level: increased dosages (to 3.2 mg/kg) merely prolonged the duration of the episode. Thus the potency of MMDA is somewhat greater than TMA and nearly three times greater than mescaline.
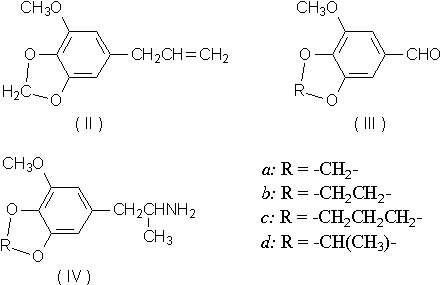
Substitution of either an ethylenedioxy or a trimethylenedioxy group for the methylenedioxy moiety (compounds IVb and IVc) resulted in a marked decrease in psychotropic effectiveness. These amphetamines were prepared from the aldehydes IIIb and IIIc by condensation with nitroethane followed by reaction with LiAlH4. A prohibitively small yield of the ethylidine analogue IIId precluded further synthetic efforts.
As with TMA, the effects of MMDA are hallucinogenic and permit complete recall. They will be reported shortly, in full, with appropriate synthetic detail.