Methylene chloride is one of the most easily available methylenating agents. It is known that methylenation with the aid of methylene chloride is performed only under pressure, as the boiling point of methylene chloride is considerably lower than the reaction temperature. Solvents of higher boiling points such as methylene bromide and iodide are also used for methylenation; methylene sulfate is used less often. We were interested in the methylenation reaction in relation to the preparation of the methylene ether of pyrocatechol, which is an intermediate in the synthesis of heliotropin.
The methylene ether of pyrocatechol is usually prepared by the interaction of pyrocatechol and methylene chloride in presence of caustic alkali, in ethanol or methanol solution at 100-115°C under pressure1-5. The yield is about 30% of the theoretical on the pyrocatechol taken.
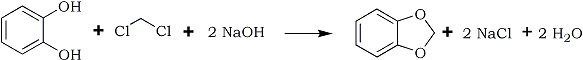
The process may be schematically represented as in this picture:
Because of the harsh reaction conditions, large amounts of solid resinous substances are formed which interfere so much with removal of the reaction products from the autoclave that this method for preparation of the methylene ether of pyrocatechol is quite unsuitable for industrial use.
Perkin, Robinson, and Thomas attempted to prepare the methylene ether of pyrocatechol without the use of pressure, by prolonged heating of pyrocatechol and methylene chloride in absolute ethanol in presence of sodium ethylate under reflux. The ether yield was low6.
A method for preparation of the methylene ether of pyrocatechol without the use of pressure is very desirable. In view of the fact that methylenation in autoclaves proceeds with the best results at 110-115°C, it was decided to use a high-boiling solvent in order to effect the reaction at a higher temperature but without the use of excess pressure. The desired result was not achieved with the use of veratrole or chlorobenzene for this purpose; the methylene ether of pyrocatechol was not isolated. On the other hand, methylenation in benzyl alcohol was successful, but the yield of pyrocatechol methylene ether did not exceed 12-13%, while part of the unconverted methylene chloride was returned. In order to decrease the return of methylene chloride and especially to increase the ether yield, we tried addition of certain metals such as copper, silver, aluminum foil, and nickel-aluminum alloy to the reaction mixture. This series of experiments, and another series in which the effect of the alkaline reagent (caustic potash, caustic soda, potassium and sodium carbonates, slaked lime, piperidine) on the course of the reaction was tested, did not give any significant results.
Considerably better results were obtained when the methylenation was effected by means of methylene chloride in presence of sodium iodide. It is known that under certain conditions sodium iodide can react with organic halogen compounds containing chlorine or bromine, chlorine or bromine in the organic compound being replaced by iodine. In this instance addition of sodium iodide to the reaction mixture increased the yield of pyrocatechol methylene ether owing to formation of methylene iodide as the result of the exchange reaction between sodium iodide and methylene chloride, as shown by the data in the table.
Table 1
Amount of Reactants (in grams) |
Temp |
Time |
Yield of Pyrocatechol Methylene Ether |
|||||
Benzyl Alcohol |
Pyrocatechol |
NaOH |
NaI |
CH2Cl2 |
Hydroquinone |
|||
292 g |
30 g |
16 g |
— |
66.0 g |
0.5 g | 122-124°C |
13.5 h |
12.1% |
595 g |
60 g |
46 g |
30 g |
90.5 g |
2.0 g | 122-124°C |
13.5 h |
31.5% |
630 g |
60 g |
39 |
20 g |
90 g |
2.0 g | 122-124°C |
8 h |
33.8% |
Notes:
* Reaction mass remained alkaline to phenolphthalein to the end of the experiment.
* Amount of caustic soda increased because the reaction mass became acid during the reaction
It has been reported previously7 that higher yields of pyrocatechol methylene ether are obtained with methylene iodide than with methylene chloride.
We found no information in the literature on the exchange reaction between methylene chloride and sodium iodide in the preparation of pyrocatechol methylene ether; it was therefore decided to study in greater detail the reaction between pyrocatechol, methylene chloride, and sodium iodide in presence of caustic alkali, in benzyl alcohol as a high-boiling solvent* without excess pressure (without the use of autoclaves)8.
* Appreciable amounts of the methylene ether of pyrocatechol could not be isolated when acetone was used as solvent for this reaction.
The question of the amount of sodium iodide to be taken for the reaction was very significant, as this influenced not only the yield but also the quality of the pyrocatechol methylene ether. Theoretically 1 mole of pyrocatechol requires 2 moles of sodium iodide. In practice it is sufficient to take 0.24 mole of sodium iodide per mole of pyrocatechol in order to obtain good quality pyrocatechol methylene ether in a yield of about 30% on the pyrocatechol taken. If the amount of sodium iodide taken for the reaction in increased, the amount of methylene iodide formed also increases, but it does not all react with pyrocatechol and some remains as an impurity in the pyrocatechol methylene ether. These two substances are almost impossible to separate by distillation, even with the aid of a very efficient column, because of the similarity of their boiling points. The yield of the ether with methylene iodide as an impurity is about 40-50%.
For isolation of methylene iodide in the pure state from the reaction products two series of experiments were carried out; in one series the reaction was effected without pyrocatechol, and in the other, without pyrocatechol and alkali. All the other conditions were the same as in the preparation of pyrocatechol methylene ether. Experiments in which methylene chloride and sodium iodide were heated in benzyl alcohol in presence of alkali gave dibenzylformal in 15.6% yield, and a small amount of methylene iodide, which could not be purified (under the same conditions but in absence of sodium iodide, dibenzylformal is formed in a lower yield, 12.5%). Methylene iodide was obtained pure in fairly good yield (45%) when methylene chloride was heated with sodium iodide in benzyl alcohol.
Simultaneously, dehydration of benzyl alcohol in presence of mineral salts resulted in the formation of dibenzyl ether (22.8%). This method for preparation of dibenzyl ether is described in the literature9. Our methods for preparation of methylene iodide and dibenzylformal differ from known methods since the reactions with methylene chloride on which they depend are effected without the use of pressure.
Perkin and Scarborough prepared methylene iodide by prolonged heating of methylene chloride with sodium iodide in acetone in a hermetically sealed vessel10. Arnhold prepared dibenzylformal by the action of heat on methylene chloride with sodium benzylate in sealed tubes at 150°C11. We found that dibenzylformal is not formed as a by-product in the preparation of pyrocatechol methylene ether under our conditions. However, if the sequence of reagent addition is changed, and pyrocatechol is added after a mixture of all the other components has been heated to the required temperature, a small amount of dibenzylformal is formed but the yield of pyrocatechol methylene ether is lowered.
The literature contains numerous references to the fact that the methylenation reaction is accompanied by oxidation processes, because the dihydroxybenzenes undergoing methylenation in an alkaline medium can absorb atmospheric oxygen. Therefore there are frequent descriptions of methylenation in an inert atmosphere or in an autoclave which must be filled as much as possible with the reagents and must contain the minimum possible volume of air. This was taken into consideration in our experiments on methylenation of pyrocatechol, and hydroquinone was tried as an antioxidant in order to diminish undesirable oxidation processes. The yields of pyrocatechol methylene ether were somewhat more stable on introduction of small amounts of hydroquinone into the reaction mixture.
Experimental
Preparation of Pyrocatechol Methylene Ether
A flask 1 liter in capacity, fitted with a mechanical stirrer, thermometer, a straight condenser, and a delivery tube reaching to the bottom of the flask, with its upper end connected to a dropping funnel, was charged with 530 g of benzyl alcohol, 32 g of caustic soda, 30 g of sodium iodide, 1 g of hydroquinone, and 30 g of methylene chloride. The reaction mixture was heated with stirring, the temperature being maintained at 122-124°C. To the mixture 120 g of methylene chloride solution in benzyl alcohol (1:1) was added from the dropping funnel, care being taken that the excess methylene chloride distilled off as slowly as possible. On the following day 14 g of caustic soda and 1 g of hydroquinone was added (the reaction must be alkaline to phenolphthalein) and the mixture was heated again at the same temperature. The total time of heating at 122-124°C was 14 hours. During the heating 31 g of methylene chloride was distilled off. The cooled reaction product was distilled in steam after it had been made alkaline by addition of caustic soda solution. During two hours about 110 g of a heavy oily product was distilled off; this was dried over sodium sulfate and distilled under vacuum through a rod-and-disk column (23 disks). The yield of pyrocatechol methylene ether was 21.4 g (32.2%); bp 63°C (18 mm); nD20 1.5400, d204 1.1972.
Evidently the pyrocatechol methylene ether still contained very small amounts of methylene iodide. According to the literature, pyrocatechol methylene ether has bp 77.5°C (27 mm)12, d0 1.2023.
Preparation of Methylene Iodide
A flask with tubes, 750 ml in capacity, fitted with a mechanical stirrer, thermometer, and reflux condenser, was charged with 258 g of benzyl alcohol, 40 g of sodium iodide, and 10 g of methylene chloride, and the mixture was heated with the temperature of the liquid at 124-126°C for 13 hours with heating. The liquid was cooled, and the crystalline precipitate was filtered off and washed with 35 g of benzyl alcohol. The combined filtrate and washings were distilled under vacuum through a rod-and-disk column (23 disks) the following fractions being collected:
- 4 g at 23-30°C (17-14 mm)
- 18.2 g at 70-83°C (13-10 mm), nD20 1.6499
- 5.9 g at 82°C (9 mm), nD20 1.5945
- 206 g at 88-92°C (9 mm)
Remaining was 73.6 g of residue consisting of a liquid and crystalline portion. The first fraction consisted of a mixture of water and a heavy, strongly refracting liquid. The second and third fractions were dried over calcium chloride and distilled in a vacuum to give 14.3 g (45%) of methylene iodide, bp 54°C (7 mm).
According to the literature, methylene iodide has bp 88-89°C (33 mm)14.
The fourth fraction was benzyl alcohol. From the residue after distillation (73.6 g),54.2 g (22.8%) of, dibenzyl ether was isolated, bp 149°C (6 mm), nD20 1.5608, d204 1.0419.
According to literature data, dibenzyl ether has bp 149-150°C (7 mm) nD20 1.5603, d204 1.045615.
Summary
- Pyrocatechol has been methylenated by means of methylene chloride in presence of sodium iodide and caustic soda in benzyl alcohol, without pressure (without use of autoclaves) for the first time. The yield of pyrocatechol methylene ether was 32% of the theoretical on the pyrocatechol taken.
- Methylene iodide was prepared in 45% of the theoretical yield on the methylene chloride taken, by the reaction of methylene chloride with sodium iodide in benzyl alcohol under atmospheric pressure.
- The reaction of benzyl alcohol, taken in excess, with methylene chloride in presence of alkali under atmospheric pressure yielded dibenzylformal; the yield was 12-15% of the theoretical on the benzyl alcohol taken.
- It is shown that dibenzylformal is not formed under the conditions described above for preparation of pyrocatechol methylene ether.