Abstract
A novel and easy to handle procedure for the regioselective rearrangement of epoxides has been developed, based on an iridium catalyst.
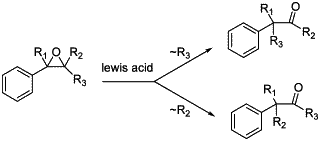
Scheme 1. Meinwald rearrangement.
Epoxides are very important and versatile intermediates in organic synthesis since they can be readily transformed into a variety of functional groups1. One of the useful synthetic routes to aldehydes and ketones from epoxides is the Meinwald rearrangement2. This reaction could be performed in the presence of various Lewis acids. The nature of the obtained product depends on the migratory aptitude of the substituents on the epoxide group but also on the nature of the Lewis acid and the solvent (Scheme 1)3,4,5,6.
The Meinwald rearrangement is usually carried out with methylaluminium bis(4-bromo-2,6-di-tert-butylphenoxide) (MABR),3 BF3·Et2O,4 MgBr2,4 lithium salts5 or indium chloride6 in stoichiometric amounts or even in excess. As some of these reactants are air-sensitive, corrosive or toxic, anhydrous conditions and inert atmosphere are generally needed.
Some examples of catalytic Meinwald rearrangements have been reported in the last decade. Kulasegaram and Kulawiec7 reported the isomerization of epoxides to carbonyl compounds with catalytic amounts of palladium species (5% mol) formed in situ from Pd(OAc)2 and a phosphine ligand. Recently, Mohan et al.8 employed a bismuth-based catalyst8b for the rearrangement of epoxides with good regioselectivities. We report in this paper the first use of an iridium species as regiospecific catalyst for the Meinwald rearrangement of epoxides under mild reaction conditions.
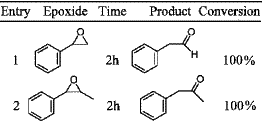
Table 1. [ Show Full Table ]
Rearrangement of epoxides with
1 mol% IrCl3·xH2O.
Iridium trichloride was used in 1% molar ratio relative to the epoxide (THF, 50°C; Table 1). Rearrangement of styrene oxide gave pure phenylacetaldehyde (entry 1). trans-β-Methylstyrene oxide and α-methylstyrene oxide (entries 2–3) rearranged selectively and quantitatively into phenylacetone and 2-arylpropanal, respectively. For trans and cis-stilbene (entries 4–5), diphenylacetaldehyde was the only formed product. In this later case, iridium lead to the aldehyde, while Pd(OAc)2, combined to PPh3, gave7a the ketone, deoxybenzoin. The only case in which IrCl3·xH2O lead to some traces of a secondary product was the reaction with dihydronaphtalene oxide (entry 6). Only 4% of cyclohexene oxide rearranges into cyclopentane carboxaldehyde (entry 7). Several tests were realized in order to improve this result by increasing the temperature to 100°C in toluene or DMF, without success. It was reported that LiBr–HMPA allowed more than 90% yield of cyclopentane carboxaldehyde in benzene at 80°C.5a
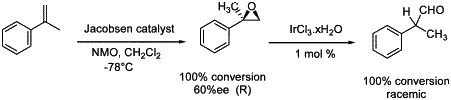
Scheme 2. Rearrangement of chiral α-methylstyrene oxide.
The optically active -arylpropanoic acid derivatives are effective nonsteroidal analgesics9 and synthetic routes to the corresponding enantiopure aldehydes are thus of high interest. As 2-arylpropanal was easily prepared by a Meinwald rearrangement (Table 1, entry 2) we focused on the chiral version. We first prepared the (R)-α-methylstyrene oxide in 60% ee with Jacobsen catalyst10 at -78°C. The rearrangement of this epoxide, was carried out with iridium trichloride in THF at 50°C but almost racemic aldehyde was obtained (Scheme 2). When the epoxide bears on the same carbon atom a methyl and a bulkier group, such as phenyl or adamantly, distinct migratory aptitudes of the two hydrogen atoms have been observed by Yamamoto et al. with monodeuteriated epoxides3. Nevertheless, the use of a bulky and chiral Lewis acid, or of a chiral iridium catalyst, may lead to a stereoselective control for this reaction.
In summary, this work presents a new effective and regioselective alternative for the Meinwald rearrangement of epoxides catalyzed by iridium(III) species. The hydrated iridium complex used is very stable and the reaction can be run under mild conditions (no inert atmosphere or high temperature are required). This alternative of the Meinwald rearrangement is also interesting since many chiral complexes of iridium are already described in the literature, and we are now focusing on the enantioselective isomerization of epoxides to carbonyl groups.
Representative procedure for the isomerization of epoxides
The epoxide (40 µL, 0.335 mmol for styrene epoxide) was added to a solution of IrCl3·xH2O (1 mg, 3.35×10-3 mmol) in THF (1 mL) and the reaction mixture stirred at room temperature. The reaction time was determined by GC analysis. After 2 h at 50°C, the solvent was removed and phenylacetaldehyde was recovered. Its purity (>99%) was determined by GC, 13C and 1H NMR. Conversions and ee values were determined by GC on a chiral column (β-dex-225 column, 30 m). All the final products were isolated in almost quantitative yields and characterized by comparison of their 13C and 1H NMR spectra with already reported data.