Procedure1,2
(1RS,2SR)-2-Diphenylphosphinoyl-1-(3,4-methylenedioxy-phenyl)-propan-1-ol
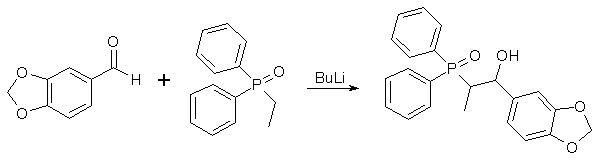
(Z)(trans)-Isosafrole
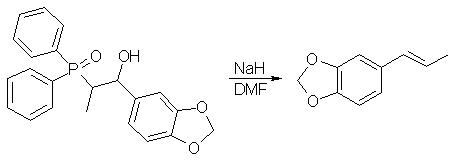
Sodium hydride (80% dispersion in oil; 24 mg, 0.79 mmol) is added in one portion to a stirred solution of the (1RS,2SR)-phosphine oxide (erythro-isomer) (300 mg, 0.79 mmol) in dry dimethylformamide (30 ml) (Note 3). The clear reaction solution is warmed to 50°C for 30 minutes by which time a white solid precipitates from solution. The raction mixture is coooled and the precipitate dissolved by the addition of water (25 ml). The mixture is diluted with brine (15 ml) and extracted with ether (3x40 ml). The combined organic extracts are washed with water (3x40 ml), dried (magnesium sulphate), and the solvent removed under reduced pressure. Bulb-to-bulb distillation (Kugelrohr apparatus) gives (Z)-isosafrole (108 mg, 84.4%) as a colourless liquid.
Notes
- All reactions in non-aqueous solutions are carried out under a nitrogen atmosphere.
- Preparation of alkyldiphenylphosphine oxides. General proceedure from phosphonium salts. Triphenyl phosphine is heated under reflux with an excess of alkyl halide. The precipitated phosphonium salt is filtered off, washed well with ether, and then heated with 30 per cent w/w aqueous sodium hydroxide (c.4 ml/g) until the benzene has distilled out. The mixture is cooled and extracted with dichloromethane, and the extracts are dried (MgSO4) and evaporated to dryness. In this way ethyldiphenylphosphine oxide is obtained from triphenyl phosphine (65.6g, 0.25 mol) and iodoethane (42.9g, 0.275 mol) in dry toluene (250 ml) to give first the phosphonium salt (103.4 g, 97.9%) after 3.5 hours, from which the phosphine oxide is obtained as needles (53.2g, 92.5%), mp 123-124°C (from ethyl acetate).
- Dry tetrahydrofuran is freshly distilled from sodium wire using benzophenone radical as an indicator. Toluene and ether are dried by distillation from sodium wire and are stored over sodium. Dimethylformamide is dried by distillation from 4A molecular sieves and stored over 4A molecular sieves.
- Not necessary if you don't mind cis/trans-isosafrole, which works just as well in all common reactions.
3,4-(Methylenedioxy)- -methylstyrene (Isosafrole)3
-methylstyrene (Isosafrole)3
To a well stirred suspension of ethyltriphenylphosphonium bromide (7.5 g, 20.2 mmol) in dry THF (100 mL), a solution of n-butyllithium (12.6 mL of a 1.6 M solution in hexane, 20.2 mmol) was added dropwise over a period of 30 min at 0 °C. The mixture was warmed to room temperature and stirred for 60 min. A solution of piperonal (3.0 g, 20.0 mmol) in THF (25 mL) was then slowly added and the resulting mixture left stirring for a further 6 h. Water (100 mL) was added, the organic layer was separated, the aqueous phase extracted twice with diethyl ether and the combined organic phases were finally dried over MgSO4. After filtration and evaporation of the solvents the residue was eluted from silica gel with CH2Cl2 to remove phosphine oxide, and the residue was purified by vacuum distillation to give 3.0 g (18.5 mmol, 92%) of a (1:10) mixture of cis- and trans-isosafrole as a colourless oil, bp 121°C/15 Torr (lit.4 bp 123°C/11.5 mmHg).