Abstract
Alkoxyl alkylthiobenzaldehydes can be readily prepared from the corresponding bromobenzaldehydes and the sodium salt of primary mercaptans in DMF.
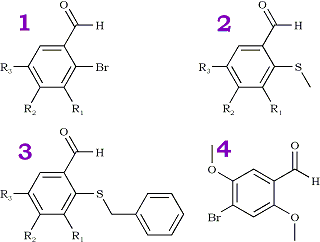
During the course of our research we required various alkoxyl alkylthio substituted benzaldehydes. Examination of the Literature revealed that classical methods1 might be suitable but cumbersome. Several workers2 have developed efficient methods for the preparation of 2-alkylthiobenzaldehydes. However, attempted preparation of alkoxyl substituted benzaldehydes using these methods resulted in extensive O-dealkylation. Thus a method for the synthesis of alkylthio substituted benzaldehydes which would accommodate the presence of alkoxyl substituents was investigated. Reaction of the known alkoxyl substituted 2-bromobenzaldehydes 1a-e with the sodium salt of primary3 mercaptans in DMF provided good to excellent yields of the alkylthiobenzaldehydes shown in Table II and Table III.
# |
R1 |
R2 |
R3 |
Ref. |
1a | H | H | OMe | |
1b | H | OMe | OMe | |
1c | H | OMe | OBn | |
1d | H | -OCH2O- | ||
1e | H | Me | OMe | |
1f | OMe | OMe | OMe | |
# |
R1 |
R2 |
R3 |
Yield |
mp (?C) |
2a |
H | H | OMe | 71% | 53-54°C (hexane) |
2b |
H | OMe | OMe | 87% |
111.5-112°C (EtOH)1 |
2c |
H | OMe | OBn | 92% | 88-89°C (hexane) |
2d |
H | -OCH2O- |
49% |
101.5-102°C (EtOH) |
|
2e |
H | Me | OMe | 86% | 61.5-62°C (hexane)2a |
The facile nature of this reaction is due presumably to the activation effect of the aldehyde group.4 Aldehydes with bromine substituted in the para position react similarly (see Table IV). However, aldehydes with bromine substituted in the meta position to the carbonyl are unreactive under these conditions.
# |
R1 |
R2 |
R3 |
Yield |
mp |
3a | H | H | OMe | 79% |
190-191.5°C (MeOH) (2,4-DNP deriv.) |
3b | H | OMe | OMe | 80% |
67.5-68°C (iPrOH)1 |
3c | H | OMe | OBn | 80% |
121.5-122°C (MeOH) |
3d | H |
-OCH2O- | 69% |
88-88.5°C (iPrOH) |
|
3e | H | Me | OMe | 73% |
155-155.5°C (MeOH) (2,4-DNP deriv.) |
# |
R |
Yield |
mp |
4a | Br | - |
See ref 10 |
4b | SMe | 89% |
94.5-95.5?C (iPrOH) |
4c | SBn | 95% |
122.5-123?C (iPrOH/hexane) |
The low yields of the methylenedioxy substituted products 2d and 3d result from cleavage of the ether by the nucleophilic sulfur reagent. In the case of the sterically congested 2-bromo-3,4,5-trimethoxybenzaldehyde (1f), reaction with the sodium salt of methyl mercaptan is very slow and affords a 80% yield of unreacted aldehyde 1f with the remainder being dehalogenated aldehyde. Reaction of 1f with the sodium salt of benzyl mercaptan gave only recovered starting aldehyde.
Although this method is limited to the use of primary mercaptans and sterically unhindered aldehydes, it offers a simple and efficient method for the preparation of oxygenated alkylthiosubstituted benzaldehydes from readily available precursors.
Experimental
General procedure:
To a stirred suspension of sodium hydride (11 mmol, 0.44g, 60% dispersion in mineral oil) in 25 ml of DMF under nitrogen atmosphere and cooled in an ice-water bath was added 11 mmol (1.31g) of benzyl mercaptan. (In the case of methyl mercaptan, the gas was passed above the DMF solution until the sodium hydride was consumed.) After the sodium hydride was consumed, the appropriate bromobenzaldehyde was added in one portion and the reaction mixture was allowed to warm to room temperature overnight. The reaction mixture was poured into 400 ml of water and the precipitated product was filtered and air dried. (In the case of 3a and 3e, the products were extracted with methylene chloride.) Crystallization of the crude products afforded pure materials.