New Formylating Agents - Preparative Procedures and Mechanistic InvestigationsPosted by Foxy2, HTML by Carboxyl[ Back to the Chemistry Archive ] European Journal of Organic Chemistry (2001), (15), 2947-2954. AbstractThe reactivity of new formylating agents related to formamide has been investigated both experimentally and theoretically. The reaction in 1,2-dichloroethane between tris(diformylamino)methane (2) and several arenes, catalyzed by AlCl3 or BCl3, was shown to proceed in good yields to afford the corresponding para-substituted aldehydes. The nature of the active electrophilic species was also investigated theoretically. Thus, the relative stability of the O- and N-protonated forms, as well as those of AlCl3 adducts, of several formylating agents - diformamide, triformamide, N,N,N,N-tetraformylhydrazine, and tris(diformylamino)methane - were determined in the gas phase and in water or DCE by means of DFT calculations at the B3LYP/6-311++G(d,p) level, the solvents being modeled with the IPCM method. The amide oxygen atom in all cases appeared to be the most basic site, both in the Brøønsted and Lewis sense, constituting a first step towards the understanding of the mechanism of this reaction.IntroductionDespite the great importance of (hetero)aromatic aldehydes as intermediates in the chemical and pharmaceutical industries, and the continuing intense research in this field, there is still a need for new synthetic methods for the introduction of the aldehyde group into (hetero)aromatics. The introduction of a formyl group by C-C bond formation is generally achieved by means of various kinds of electrophilic aromatic substitutions, which can be subdivided further into two types of reactions: (a) reactions involving the acid-promoted generation of a formyl cation or a precursor thereof, such as formyl fluoride/boron trifluoride (Olah formylation),[1] CO/HCl (Gattermann- Koch reaction),[1,2] CO/HF,[1] HCN/HCl (Gattermann reaction),[3] and the Vilsmeier reagent,{1} formed from disubstituted formamides and phosphorus oxychloride or phosgene, and (b) reactions yielding primary products which are immediately oxidized to aldehydes, such as the synthesis of salicylaldehydes from phenols and paraformaldehyde in the presence of SnCl4/trioctylamine[1,4] or the Duff reaction, yielding aromatic aldehydes from activated aromatic compounds and urotropine in the presence of acids.[1,5]
Nowadays, the Vilsmeier (or Vilsmeier-Haack) reaction is the most common method for formylation of aromatic rings. Nevertheless, it, like the other methods, has some significant disadvantages. The formylating agents in these reactions are often toxic (as in the cases of carbon monoxide, hydrogen cyanide, formyl fluoride, and phosgene), and they are also difficult to handle in large amounts. Furthermore, the scope of these reactions is limited. The Vilsmeier-Haack reaction is the method with the widest scope, but it is incapable of formylating simple alkylarenes (unless they are much more reactive than benzene). A further difficulty is the occurrence of phosphorus compounds in wastewater, which is a serious environmental problem, and the formation of highly toxic, carcinogenic dialkylcarbamoyl chloride in a side reaction. The dichloromethyl methyl ether/Lewis acid formylating system has an even wider scope than the Vilsmeier-Haack reagent, but it has not yet found practical use.[1,3,6]
Formamide Derivatives as Formylating AgentsSome time ago, we reported on a new synthetic method for the formylation of (hetero)aromatic compounds using triformamide (1)/AlCl3 as the formylating system (Scheme 1).[7,8] This reaction is useful for the formylation of a wide range of substrates, including unsubstituted and alkyl-substituted aromatics, aromatic ethers, tertiary aromatic amines, fused aromatic rings, and thiophenes.
Scheme 1. New formylating agents based on formamide derivatives
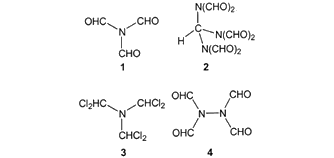
1. Triformamide In this paper we wish to introduce some other reagents based on formamide derivatives that can be used to formylate (hetero)aromatic compounds. We also present results of computational investigations aimed at elucidation of the reaction intermediates of these formylations.
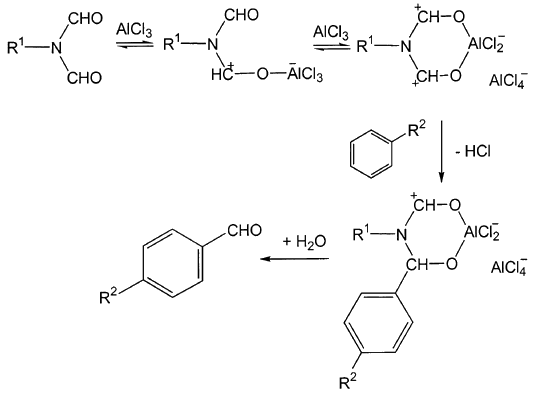
The typical mechanism of electrophilic aromatic substitution reactions involves, as a preliminary step, the formation of the actual electrophilic species. In the case of amides, it is assumed that the Lewis basic site is the oxygen atom, by analogy with the behavior towards protonation.[9] However, there is no guarantee that the behavior towards the proton exactly matches that towards other Lewis acids. Thus, although one can easily formulate the generation of the electrophilic species as a Lewis acid/base process involving the amide oxygen atom as the basic site, the actual nature of the active species must be elucidated independently. For this purpose, we have carried out quantum chemical calculations aimed at quantitative determination of the extent of preference of formylating agents for protonation or Lewis acid complexation at either the oxygen or nitrogen atom in the amide group.
Formylations with Tris(diformylamino)methaneDuring the preparation of triformamide (1),[11,12] tris(diformylamino)methane[10] (2) is formed as a by-product.[8] In an improved method, 2 can be prepared from triformamide and sodium diformamide[13,14] in 80% yield.[8,15] Tris(diformylamino)methane (2) contains six formyl groups, plus an additional one masked as an orthoamide function, which seems to set a record for this type of compounds. In the presence of (strong) Lewis acids, it is capable of formylating activated arenes.[8,15] The efficiency of 2 as a formylating agent is higher than that of triformamide (1), since up to three out of the seven formyl groups in 2 can be used synthetically, whereas 1 can transfer only one of its three formyl groups.
In the presence of AlCl3 or BCl3, alkylarenes and aromatic ethers are formylated by tris(diformylamino)methane (2) in moderate to good yields (see Table 1). With AlCl3 as the Lewis acid, yields are highest if a tris(diformylamino)methane/AlCl3 ratio of 1:6 to 1:8 is used (see Table 2). This corresponds to a ratio of 1:2 with regard to the number of formyl groups transferred, a value coincident with the ratio found in the Friedel-Crafts acylation of esters and anhydrides. 1,2-Dichloroethane proves to be the best solvent for the reaction, although other solvents, such as chlorobenzene and carbon disulfide, are also suitable. Solvents containing nitro groups, such as nitromethane and nitrobenzene, seem to suppress the formylating reaction, probably because of complexation of the Lewis acid.
Notes[a] Molar ratio substrate/2/Lewis acid (3:1:6). Table 2. Influence of Molar Ratio of Reagents on Yield of Formylation of o-Xylene with Tris(diformylamino)methane (2) in the Presence of AlCl3 (Procedure I)
Notes[a] With respect to 2.[b] Product is 3,4-dimethylbenzaldehyde. Yield calculated for transfer of 3 formyl groups per molecule 2. [c] Aged AlCl3 (partially hydrolyzed by repeated opening of the container). [d] 1,2-Bis(3,4-dimethyphenyl)ethane (3 %) was obtained as a by-product. [e] Reaction performed without additional solvent. The reaction features a high para selectivity in the substrate, consistent with the high steric bulk of the attacking species. As can be appreciated from Table 1 and Table 2, in order to achieve satisfactory yields with formamide derivatives bearing one or more diformylamino groups, it is necessary to use at least 2 equiv. of Lewis acid. It is hence very likely that the primary adduct reacts with further Lewis acid to form the formylating species. Lastly, we emphasize that the method we have presented can be performed using only stoichiometric amounts of the formamide derivative, and 2 equiv. of Lewis acid per formyl group transferred. E-factors (kg of waste per kg of product) of the new formylation procedures are in the range from 4 to 11; further investigations will show whether these values can be reduced by use of other types of catalysts. The comparable method with dichloromethyl methyl ether often employs a large excess of the formylating mixture (up to 18 equiv.),[16,17] and the regioselectivity is rather low.[18] Formylation with Other Formamide DerivativesThe perchlorinated derivative of triformamide, tris(dichloromethyl)amine (3),[12] can be prepared from N,N-dimethylformamide and phosgene, followed by photochlorination of the resulting N,N-dimethylformamide chloride. In the presence of various Lewis and Brøønsted acids, 3 also proves to be a good formylating agent, and it is suitable for the formylation of a wide range of aromatic compounds.[8] Thus, toluene reacts with 3/AlCl3 to give tolualdehyde, in a yield of 70% and a p/o ratio of 30:1. Similarly, anisaldehyde can be prepared from anisole and 3/ZnCl2 in 62% yield (p/o 24:1) (see Exp. Sect.). However, this complex subject will be dealt with in more detail in a forthcoming paper. Preliminary studies on the formylating ability of another formamide derivative, N,N,N,N-tetraformylhydrazine (4), are also encouraging.[19,20] ConclusionArene formylation can conveniently be carried out in satisfactory yields with formamide derivatives in the presence of Lewis acids. Quantum chemical calculations provide cogent indications of the nature of the electrophilic species involved in this reaction. Thus, in the attack by a proton or by AlCl3 (protonation or Lewis acid adduct formation, respectively), the amide oxygen atom is the most basic site, both in the gas phase and in water or DCE as solvents. The formation of a chelate complex between the two adjacent carbonyl oxygen atoms in a diformamide group and AlCl3 is also supported. All these results reinforce the proposition that the attacking electrophilic species is formed by attachment of the Lewis acid to the oxygen atom of the formamide derivatives, and endorse the proposed reaction mechanism (Scheme 5).
ExperimentalFormylation of Toluene with 2 and Various Amounts of AlCl3. (Typical) Procedure I: Dry AlCl3 (90 mmol) was added with stirring to a mixture of toluene (45 mmol) and dry 1,2- dichloroethane (25 mL), cooled in an ice/salt bath. After a few minutes, tris(diformylamino)methane (2) (15 mmol) was added. The reaction mixture was stirred in the cooling bath with exclusion of moisture for 20 h, during which the temperature rose to 0 °C. The viscous, reddish brown mixture was carefully hydrolyzed by addition of 100 mL of ice-cold water, and steam-distilled. The organic layer of the distillate was separated, and the aqueous phase extracted three times with 10 mL of 1,2-dichloroethane. The combined organic layers were dried with sodium sulfate and filtered, and the solvent was evaporated at ordinary pressure. p- Tolualdehyde (b.p. 84 °C/12 Torr) (204-205 °C/760 Torr [26]) was isolated by fractional vacuum distillation. Yield 25 mmol (55%). Formylation of Aromatic Compounds with 2/Lewis Acid (General) Procedure II: A stirred mixture of the aromatic compound (40-60 mmol) and 1,2-dichloroethane (25-40 mL) was cooled in an ice/salt bath. The Lewis acid was added first, over 5 min, followed by tris(diformylamino)methane (2). During the reaction time stated, the temperature of the reaction mixture rose from ca. -15 °C to 0 °C (see Table 1). After hydrolysis by careful addition of 100 mL of water, the organic layer was separated and the aqueous phase was extracted three times with 10 mL of 1,2-dichloroethane. Workup A: The organic layers were combined, and the solvent was evaporated at reduced pressure. The residue was treated with 100 mL of a saturated sodium hydrogen sulfite solution, 3 mL of methanol, and ca. 250 mg of tetrabutylammonium hydrogen sulfate. The bisulfite adduct separated upon stirring and was isolated by filtration. The adduct was cleaved by addition of either 100 mL of 8% NaHCO3 solution or 20 mL of 10% HCl. The aldehyde was extracted from the mixture three times with 10 mL of ether. The combined organic layers were dried with sodium sulfate and filtered, and the solvent was evaporated. The aldehyde was obtained by fractional distillation of the residue through a 15-cm Vigreux column. Workup B: The combined organic layers were dried with sodium sulfate and filtered, and the solvent was evaporated at reduced pressure. The aldehyde was obtained by fractional distillation of the residue through a 10-cm Vigreux column. Formylation of Aromatic Compounds with 3/Lewis Acid. (a) Tolualdehyde from 3 and AlCl3: Compound 3 (7.3 g, 30 mmol) was added over 10 min, at 5 °C and with stirring, to a cooled mixture of dry toluene (25 g, 0.27 mmol) and AlCl3 (11 g, 80 mmol). The cooling bath was removed and the mixture was stirred for 15 h at 20 °C. The reaction mixture was hydrolyzed with 50 mL of water and steam-distilled. The organic phase of the distillate was separated and the aqueous phase was extracted three times with CH2Cl2. The organic phases were combined and dried with sodium sulfate. The drying agent was removed by filtration. The solvents were removed from the filtrate by distillation at ordinary pressure. Tolualdehyde (2.31 g, 70%; 97% p-isomer, 3% o-isomer) was isolated by distillation (b.p. 80 °C/12 Torr). (b) Anisaldehyde from 3 and ZnCl2: ZnCl2 (2.18 g, 31.6 mmol) was added to a solution of 3 (4.2 g, 15.8 mmol) in anisole (20 mL, 187 mmol), whereupon the mixture turned red. The mixture was heated with stirring for 3 h at 60-65 °C, hydrolyzed with 100 mL of water, and steam-distilled. Dichloromethane (30 mL) was added to the distillate in order to produce better phase separation. The organic phase was separated and the aqueous phase was extracted three times with 50 mL of dichloromethane. The combined organic phases were dried with sodium sulfate. The solvents were evaporated, and the residue was distilled in vacuo through a 20-cm Vigreux column. Yield: 1.33 g (62%) anisaldehyde (96% p-isomer, 4% o-isomer), b.p. 80 °C/0.01 Torr. References[1] G. Simchen, Methoden Org. Chem. (Houben-Weyl), 1983, vol. E3. |