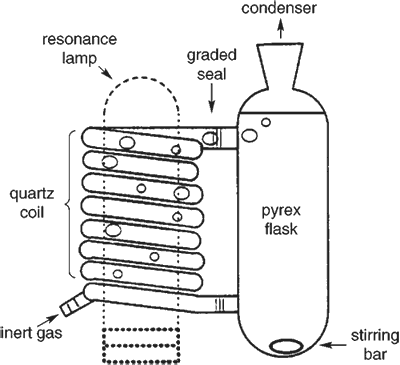
Figure 1.
Photochemical reactor.
Photochemical reactions can be conveniently performed at a variety of wavelengths—such as 214 (Zn), 229 (Cd) and 254 nm (Hg)—utilizing ~25 watt spectra calibration lamps. Switching wavelengths by switching lamps can favorably increase yields. For example, the product ratio in a sterically congested stilbene isomerization increased from 1:5 to 1:1 with a change in lamp1.
With the Phillips line of lamps, the lamp must be oriented vertically with its base down during operation. Quartz reactors are necessary to transmit the light at these wavelengths2, however, most simple reactor designs utilize Pyrex materials2. The pictured reactor (see Figure 1) accommodates both the orientation of the Phillips lamp and requirement for quartz materials. Serendipitously, this design creates a generally useful, easily assembled device for laboratory scale reactions. Utilizing a Pyrex reservoir with graded seals and quartz tubing for the light-transmitting component allows construction of the device by a person with only modest glassblowing skills. The lamp is surrounded by the sample, so light is efficiently absorbed. In addition, no coating of the inner reactor walls, which is commonly seen in immersion well reactors, has been observed. Since the resonance lamps are small, they do not require water-cooling. Materials for the device include a 120-cm × 8-mm o.d. quartz tube (~$15), two 8-mm o.d. quartz to Pyrex graded seals (~$18 each), a T/S 24/40 joint (~$5), and miscellaneous pieces of Pyrex glass. Shaping of the 120-cm × 8-mm o.d. tube into the quartz coil would cost about $75 if made by a glass-blower.
The lamp is inserted up into the quartz coil, which may be surrounded with aluminum foil to increase efficiency. Convection circulates enough air to cool the reactor if the foil cylinder is kept large. Rising bubbles of slowly flowing, inert gas move into the reactor through a piece of Pyrex tubing connected to a short quartz nipple by a minimum length of Teflon tubing (see Figure 1). This flow of inert gas removes oxygen and circulates the solution through the reactor without evaporating excessive amounts of solvent. In principle, the reservoir could be very large. We have used up to 150 mL. We routinely convert 0.5 g of di-tert-butylstilbene in hexane solution to near its photostationary state in 10 hours with a cadmium resonance lamp.