Small-scale reactions performed at reflux temperatures invariably use water-cooled condensers1. Cooling water from municipal suppliers is particularly prone to variations in both temperature and pressure, with potentially detrimental consequences2. Our effort to overcome this problem led us to develop a simple, cost-effective, self-circulating system that uses convection to cool a reflux condenser.
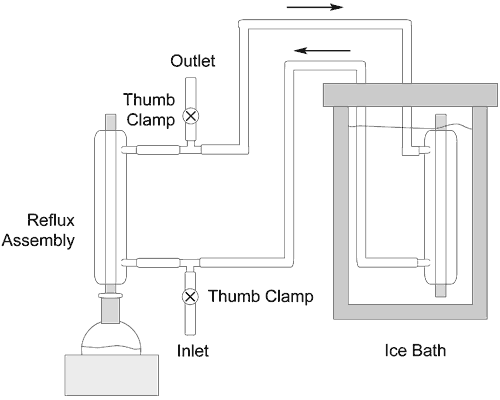
Figure 1.
Diagram of the cooling system.
The technique entails connecting the reflux condenser in parallel with another condenser that is immersed in an ice-bath (Fig. 1). The reflux condenser inlet and outlet are each connected to a T-connector and are linked together by a section of tubing about a meter in length. This tubing is connected to a second condenser that is placed inside a two-liter insulated flask (cooler). Efficient cooling requires the complete exclusion of air bubbles, which is readily achieved by filling the system with water through the lower T-connector. When the system is completely filled with water the inlet and outlet tubing is sealed with a thumb clamp and the insulated bath is then filled with ice.
We have experienced uniformly excellent results with this cooling system. Refluxing reactions have been cooled over extended periods (24–48 hours) for solvent volumes as low as 10 mL to as high as 250 mL in a variety of solvents (THF, acetone, ethyl acetate, chloroform, and water). Independent testing of this system established that the cooling method is effective for both refluxing reactions and Soxhlet extraction. The method tolerates different tube lengths (1–2 m) and height differences (±15 cm) between the cooler and the condenser. The only imperative is the complete exclusion of air bubbles and the use of tightly fitting tubing that does not leak. The cooling is remarkably efficient and offers several advantages over conventional water circulation: flooding is effectively prevented, water shut-off systems are not necessary, the equipment is inexpensive, and the use of large volumes of water is avoided.