dl-Ephedrine
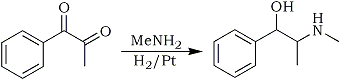
A mixture of 50 cc. of absolute ethyl alcohol, 7.4 g. of methylphenyl diketone (1/20 mole) and an alcoholic solution of methylamine containing 1.6g (1/20 mole) was reduced catalytically with hydrogen in the presence of 0.1 g. of platinum oxide2. In some experiments there was a long induction period and then the yield was low. This behavior could be obviated by reducing the catalyst first and then adding the reactants. When reduction no longer proceeded, the catalyst was removed by filtration and about half the alcohol removed under reduced pressure. By this means any excess of methylamine was removed. The solution was made just acid with alcoholic hydrogen chloride and evaporated to dryness. The solid hydrochloride was washed with cold acetone and dried. A small amount of pseudoephedrine hydrochloride could be extracted from this by means of hot chloroform and by working up a number of extractions sufficient was obtained for definite identification (mp 164°C; free base, mp 118°C)1,2.
The dl-ephedrine hydrochloride was purified by recrystallizing once from alcohol-acetone and melted at 189°C1. The free base was recrystallized from chloroform-petroleum ether and melted at 75°C. The yield was 2.5-4.0 g.