This procedure, to use ethylenediammonium diacetate as a Henry reaction catalyst was originally suggested by Osmium, and the following success rates was gathered from various researchers who wishes to remain anonymous.
Preparation of Ethylenediammonium diacetate (EDDA)
A 150 ml beaker, containing 100 ml dry ether and 12.0 g (0.2 mol) of ethylenediamine, is placed in an ice-bath and a solution of 24.0 g (0.4 mol) glacial acetic acid in 20 ml ether is added with stirring at such a rate as to prevent boiling of the ether. The solution is left to crystallize overnight, then filtered with suction, the crystals washed with ether and recrystallized from approximately 50 ml MeOH. Yield after drying in a vacuum desiccator is around 27.5g (75%) of colorless needles, mp 114°C.
| Product | Yield |
| Phenyl-2-nitropropene | 60% |
| 4-Fluorophenyl-2-nitropropene | 5% |
| 4-(Trifluoromethyl)-phenyl- 2-nitropropene |
3%4 |
| 4-Methoxyphenyl-2-nitropropene | 71% |
| 4-Methylthiophenyl-2-nitropropene | 57%1 |
| 2,5-Dimethoxyphenyl-2-nitropropene | 50% |
| 2,5-Dimethoxyphenyl-2-nitroethene | 95% |
| 2,5-Dimethoxy-4-Methyl- phenyl-2-nitropropene |
80% |
| 2,5-Dimethoxy-4-Methyl-nitrostyrene | 88% |
| 2,5-Dimethoxy-4-Ethyl-nitrostyrene | 84% |
| 3,4-Methylenedioxyphenyl- 2-nitropropene |
14%1,2 |
| 2,4,6-Trimethoxyphenyl-2-nitropropene | 67% |
| 2,4,5-Trimethoxyphenyl-2-nitropropene | 58% |
| 1-(3-Indolyl)-2-nitropropene | 37%3 |
Notes
- This nitrostyrene is unstable, and should
thus be stored in the fridge. - An unknown voluminous white precipitate
was also formed. A batch using 10 g
piperonal gave ca 4.5 g of this material.
Further investigation is warranted. - The aldehyde is pretty insoluble in IPA,
the use of THF as a co-solvent might
increase the yield somewhat. - A reaction time of 14 days at -10°C
gave 3%, standard conditions
gave a maximum of 1.5% yield.
Preparation of substituted nitrostyrenes
General Procedure
50 mmol of the substituted benzaldehyde, 60 mmol of nitroalkane and 5 mmol of ethylenediammonium diacetate is dissolved in 25-50 ml of iPrOH with stirring (with gentle heating if required to dissolve all solids) and the solution is left to stir at room temp for 24 h, whereafter the formed nitrostyrene is allowed to crystallize in the freezer for 12 h. The precipitate is then filtered off, washed with a small amount of iPrOH, sucked as dry as possible at the pump and air dried. The yields are typically 60-70%, with the exception of the fluorinated substrates, as well as for piperonal, the latter due to the formation of a large amount of a byproduct of unknown composition.
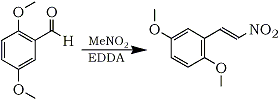
2,5-Dimethoxynitrostyrene
The procedure has been optimized for the preparation of 2,5-Dimethoxynitrostyrene, and the yield is typically very high for this product, usually exceeding 95%.