Introduction
As the supply of GBL quickly diminishes, the desire to find a synthesis of GBL from sources such as tetrahydrofuran (THF) and 1,4-butanediol (BDO) increases.
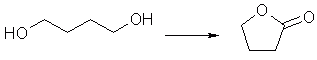
Just to make this a more thorough study, here are a few more ref's for a few more procedures worth looking at. I think you'll like them; the oxidation of butanediol into butyrolactone is a classic, and is the basis of a huge number of studies. Enjoy. Gratis a Beilstein magnificum.
Reactant butane-1,4-diol (BRN 1633445)
Product dihydro-furan-2-one (BRN 105248)
Reaction 1 (Copper chromite)
Temperature: 200 C
Other Conditions: Erhitzen mit einem
Chrom(III)-oxid enth. Kupfer-Katalysator
Ref. 1 605477; Journal; Reppe et al.; JLACBF; Justus Liebigs Ann. Chem.; 596; 1955; 1, 158, 178;
Reaction 2 (Copper oxide)
Catalyst: CuO
Time: 15 hours
Temperature: 200 C
Yield 80%
Note 1 Further byproducts given
Ref. 1 5549295; Journal; Berthon, Bruno;
Forestiere, Alain; Leleu, Gerard; Sillion, Bernard; TELEAY; Tetrahedron
Lett.; FR; 22; 41; 1981; 4073-4076;
Reaction 3 (Sodium bromite)
Reagent: NaBrO2*3H2O, NaHCO3, 4-(benzyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl
Solvent: CH2Cl2
Time: 3 hours
Other Conditions: Ambient temperature
Yield 95%
Ref. 1 5504352; Journal; Inokuchi, Tsutomo; Matsumoto, Sigeaki; Nishiyama,
Tokio; Torii, Sigeru; JOCEAH; J.Org.Chem.; EN; 55; 2; 1990; 462-466;
Reaction 4 (Sodium bromite)
Reagent: NaBrO2*3H20, Al2O3
Solvent: CH2Cl2
Other Conditions: Ambient temperature
Yield 92%
Ref. 1 5895926; Journal; Morimoto, Takashi; Hirano, Masao; Iwasaki, Keiko; Ishikawa, Takashi; CMLTAG; Chem.Lett.; EN; 1; 1994; 53-54;
Reaction 5 (Sodium bromite)
Reagent: sodium bromite
Solvent: H2O acetic acid
Time: 10 hours
Other Conditions: Ambient temperature
Yield 92%
Ref. 1 5666548; Journal; Kageyama, Toshifumi; Kawahara, Shuji; Kitamura, Kohji; Ueno, Yoshio; Okawara, Makoto; CMLTAG; Chem.Lett.; EN; 1983; 1097-1100;
Reaction 6 (Sodium bromate)
Reagent: aq. NaBrO3, aq. NaHSO3
Solvent: acetonitrile
Time: 2 hours
Other Conditions: Heating
Yield 62%
Ref. 1 6008056; Journal; Takase, Kiyoshi; Masuda, Haruyoshi; Kai, Osamu; Nishiyama, Yutaka; Sakaguchi, Satoshi; Ishii, Yasutaka; CMLTAG; Chem.Lett.; EN; 10; 1995; 871-872;
Reaction 7 (Sodium bromate)
Reagent: aq. sodium bromate
Catalyst 47% hydrobromic acid
Solvent: CCl4
Time: 5 hours
Temperature: 37 C
Yield 78%
Ref. 1 5934286; Journal; Kajigaeshi, Shoji; Nakagawa, Takashi; Nagasaki, Noritaka; Yamasaki, Hiromochi; Fujisaki, Shizuo; BCSJA8; Bull.Chem.Soc.Jpn.; EN; 59; 3; 1986; 747-750;
Reaction 8 (Peracetic acid, sodium bromide)
Reagent: AcOOH/NaBr
Solvent: ethyl acetate
Time: 2 hours
Temperature: 39.9 C
Ref. 1 5647691; Journal; Morimoto, Takashi; Hirano, Masao; Hamaguchi, Takayoshi; Shimoyama, Masahide; Zhuang, Xiumin; BCSJA8; Bull.Chem.Soc.Jpn.; EN; 65; 3; 1992; 703-706;
Reaction 9 (Potassium dichromate)
Reagent: potassium dichromate
Solvent: dimethylformamide
Time: 20 hours
Other Conditions: Ambient temperature
Yield 70%
Ref. 1 5611749; Journal; Kim, Kwan Soo; Szarek, Walter A.; CRBRAT; Carbohydr.Res.; EN; 104; 1982; 328-333;
Reaction 10 (Potassium permanganate)
Reagent: KMnO4, CuSO4*5H2O
Solvent: CH2Cl2
Yield 58%
Ref. 1 5932996; Journal; Jefford, Charles W.; Wang, Ying; JCCCAT; J.Chem.Soc.Chem.Commun.; EN; 10; 1988; 634-635;
Reaction 11 (Hydrogen peroxide)
Reagent: 35% H2O2
Catalyst tricetylpyridinium-12-tungstophosphate
Solvent: 2-methyl-propan-2-ol
Time: 24 hours
Temperature: 82 C
Other Conditions: Heating
Note 1 Yield given
Ref. 1 5740601; Journal; Yamawaki, K.; Nishihara, H.; Yoshida, T.; Ura, T.; Yamada, H.; et al.; SYNCAV; Synth.Commun.; EN; 18; 8; 1988; 869-876;
Reaction 12 (Hydrogen peroxide)
Reagent: 35% aq. hydrogen peroxide, tris(cetylpyridinium) 12-tungstophosphate (CWP)
Solvent: 2-methyl-propan-2-ol
Time: 24 hours
Other Conditions: Heating
Yield 97%
Ref. 1 5639938; Journal; Ishii, Yasutaka; Yamawaki, Kazumasa; Ura, Toshikazu; Yamada, Hiroshi; Yoshida, Tsutomu; Ogawa, Masaya; JOCEAH; J.Org.Chem.; EN; 53; 15; 1988; 3587-3593;
Reaction 13 (Hydrogen peroxide)
Reagent: tris(cetylpyridinium) 12-tungstophosphate (CWP), 35% H2O2
Solvent: 2-methyl-propan-2-ol
Time: 24 hours
Other Conditions: Heating other $a,$w-diols; var. solvents and reagents
Ref. 1 5639734; Journal; Ishii, Yasutaka; Yoshida, Tsutomu; Yamawaki, Kazumasa; Ogawa, Masaya; JOCEAH; J.Org.Chem.; EN; 53; 23; 1988; 5549-5552;
Reaction 14 (Cyclohexanone)
Reagent: cyclohexanone
Catalyst RuHClCO(PPh3)3
Time: 20 hours
Temperature: 140 C
Other Conditions: hydrogen transfer; further carbonyl compounds, further ruthenium(II) catalysts
Ref. 1 5769676; Journal; Marcec, Radovan; ZNBAD2; Z.Naturforsch.B Anorg.Chem.Org.Chem.; EN; 39; 12; 1984; 1823-1824;
Reaction 15 (Acetone)
Reagent: acetone
Catalyst RuH2(PPh3)4
Solvent: toluene
Time: 3 hours
Temperature: 180 C
Yield 100%
Ref. 1 5554152; Journal; Murahashi, Shun-Ichi; Ito, Kei-ichiro; Naota,
Takeshi; Maeda, Yoshihiro; TELEAY; Tetrahedron Lett.; EN; 22; 52; 1981; 5327-5330;
Reaction 16 (Exotic ruthenium catalyst)
Catalyst <(2,2'-bipyridine)(2,2':6',2''-terpyridine) RuO>2+ - complex
Time: 20 hours
Other Conditions: Ambient temperature buffer; electrooxidation: 56 mA current
Yield 44%
Ref. 1 5532772; Journal; Navarro, Marcelo; Giovanni, Wagner F. De; Romero, Jose R.; TETRAB; Tetrahedron; EN; 47; 4/5; 1991; 851-857;
Reaction 17 (Exotic ruthenium catalyst)
Reagent: RuH3(Ph3P)3
Solvent: benzene
Time: 24 hours
Temperature: 40 C
Yield 85%
Ref. 1 5645533; Journal; Lin, Yingrui; Zhu, Xianchao; Zhou, Yuefen; JORCAI;
J.Organomet.Chem.; EN; 429; 2; 1992; 269-274;
Reaction 18 (t-butyl hydroperoxide)
Reagent: tert-butyl hydroperoxide, 3A molecular sieves
Catalyst Zr(O-n-Pr)4
Solvent: CH2Cl2
Time: 2 days
Temperature: 60 C
Yield 50%
Ref. 1 6012392; Journal; Krohn, Karsten; Vinke, Ingeborg; Adam, Horst;
JOCEAH; J.Org.Chem.; EN; 61; 4; 1996; 1467-1472;
Reaction 19 (Na2HPO4)
Reagent: benzyltrimethylammonium tribromide, Na2HPO4
Solvent: CCl4
Time: 5.5 hours
Temperature: 70 C
Yield 60%
Ref. 1 5936936; Journal; Kajigaeshi, Shoji; Kawamukai, Hiroshi; Fujisaki, Shizuo; BCSJA8; Bull.Chem.Soc.Jpn.; EN; 62; 8; 1989; 2585-2588;
Reaction 20 (Exotic reagent)
Reagent: tris(cetylpyridinium) peroxo-12-molybdophosphate (PCMP)
Solvent: benzene
Time: 2 hours
Other Conditions: Heating
Yield 66%
Ref. 1 5952228; Journal; Ishii, Yasutaka; Yamawaki,
Kazumasa; Yoshida, Tsutomu; Ura, Toshikazu; Ogawa, Masaya; JOCEAH; J.Org.Chem.;
EN; 52; 9; 1987; 1868-1870;
Reaction 21 (Phenyl bromide)
Reagent: Phenyl bromide, K2CO3
Catalyst Pd(OAc)2, Triphenylphosphine
Solvent: 1,2-dimethoxy-ethane
Time: 12 hours
Temperature: 85 C
Yield 74%
Ref. 1 5579141; Journal; Tamaru, Yoshinao; Yamada, Yoshimi; Inoue, Kenji; Yamamoto, Youichi; Yoshida, Zen-ichi; JOCEAH; J.Org.Chem.; EN; 48; 8; 1983; 1286-1292;
Reaction 22 (Exotic chromium reagent)
Reagent: (bipy)H2CrOCl5
Solvent: CH2Cl2
Time: 4 hours
Other Conditions: Ambient temperature
Yield 95%
Ref. 1 5574719; Journal; Chakraborty, T. K.; Bhushan, Vidya; Chandrasekaran, S.; IJSBDB; Indian J.Chem.Sect.B; EN; 22; 1; 1983; 9-11;
Reaction 23 (Exotic ruthenium catalyst)
Reagent: allyl methyl carbonate
Catalyst RuH2(PPh3)4
Solvent: toluene
Time: 3.5 hours
Other Conditions: Heating
Yield 79%
Ref. 1 5611107; Journal; Minami, Ichiro; Tsuji, Jiro; TETRAB; Tetrahedron; EN; 43; 17; 1987; 3903-3916;
Reaction 24 (Exotic reagent)
Reagent: 4-methoxy-1-oxo-2,2,6,6-tetranethylpiperidinium chloride
Solvent: CH2Cl2
Time: 10 min
Temperature: 25 C
Yield 81%
Ref. 1 5699448; Journal; Miyazawa, Takeo; Endo, Takeshi; JOCEAH; J.Org.Chem.; EN; 50; 20; 1985; 3930-3931;
Reaction 25 (Exotic ruthenium catalyst)
Reagent: benzalacetone, Et3N
Catalyst Ru2Cl4((-)-DIOP)3
Time: 5 hours
Temperature: 190 C
Yield 81%
Ref. 1 5608240; Journal; Ishii, Youichi; Osakada, Kohtaro; Ikariya, Takao; Saburi, Masahiko; Yoshikawa, Sadao; CMLTAG; Chem.Lett.; EN; 1982; 1179-1182;
Reaction 26 (Oxygen/palladium)
Reagent: oxygen
Catalyst Pd on Na-ZSM-5 zeolite
Solvent: various solvents
Time: 24 hours
Temperature: 117.9 C
Yield 81%
Ref. 1 5914130; Journal; Baba, T.; Kameta, K.; Nishiyama, S.; Tsuruya, S.; Masai, M.; JCCCAT; J.Chem.Soc.Chem.Commun.; EN; 15; 1989; 1072-1073;
Reaction 27 (Trichloromelamine)
Reagent: trichloromelamine
Solvent: CH2Cl2
Time: 12 hours
Other Conditions: Ambient temperature
Yield 87%
Ref. 1 5815102; Journal; Kondo, Shuji; Ohira, Mari; Kawasoe, Shinya; Kunisada, Hideo; Yuki, Yasuo; JOCEAH; J.Org.Chem.; EN; 58; 18; 1993; 5003-5004;
Reaction 28 (N-chlorosuccinimide)
Reagent: N-chlorosuccinimide
Solvent: CH2Cl2
Time: 5 hours
Temperature: 20 C
Yield 88%
Ref. 1 5957676; Journal; Kondo, Shuji; Kawasoe, Shinya; Kunisada, Hideo; Yuki, Yasuo; SYNCAV; Synth.Commun.; EN; 25; 5; 1995; 719-724;
Reaction 29 (N-iodosuccinimide)
Reagent: N-iodosuccinimide, silver acetate
Solvent: benzene
Time: 7.5 hours
Other Conditions: Heating
Yield 89%
Ref. 1 5747330; Journal; Beebe, Thomas R.; Adkins, Rick; Baldridge, Ruth; Hensley, Vivian; McMillen, Doug; et al.; JOCEAH; J.Org.Chem.; EN; 52; 24; 1987; 5472-5474;
Reaction 30 (Oxygen/palladium)
Reagent: O2
Catalyst Pd%0&, K-L zeolite
Solvent: various solvents
Time: 24 hours
Temperature: 83.9 - 117.9 C
Pressure 757.6 Torr
Yield 91.1%
Other Conditions: other supporting zeolites, other solvents, other temperatures
Ref. 1 5506788; Journal; Baba, Toshihide; Kameta, Keiichiro; Nishiyama, Satoru; Tsuruya, Shigeru; Masai, Mitsuo; BCSJA8; Bull.Chem.Soc.Jpn.; EN; 63; 1; 1990; 255-257;
Reaction 31 (Exotic chromium reagent)
Reagent: (1,10-phenanthroline)H2CrOCl5
Solvent: CH2Cl2
Time: 8 hours
Temperature: 25 C
Yield 95%
Ref. 1 5555229; Journal; Chakraborty, T. K.; Chandrasekaran, S.; TELEAY; Tetrahedron Lett.; EN; 21; 1980; 1583-1586;
Reaction 32 (Exotic reagent)
Reagent: 4-(benzoyloxy)-2,2,6,6-tetramethylpipe ridine-1-oxyl, NaBr
Solvent: CH2Cl2
Other Conditions: NaHCO3-buffered at pH 8.6; electrolysis
Yield 97%
Ref. 1 5584616; Journal; Inokuchi, Tsutomu; Matsumoto, Sigeaki; Torii, Sigeru; JOCEAH; J.Org.Chem.; EN; 56; 7; 1991; 2416-2421;
Reaction 33 (Acetobacter culture)
Other Conditions: mit Hilfe von Acetobacter-Kulturen
Ref. 1 605476; Patent; Weinessigfabr. A. Enenkel; DE 929543; 1949;
Reverend Drone