Previously the isolation of three compounds with hypnotic potentiating activity from the volatile oil of Acorus calamus L., lndian, had been reported1. Two of these proved to be the cis-trans isomers of 1,2,4-trimethoxy-propenylbenzene, namely asarone (α-asarone) and β-asarone (II and I).
The cis-trans relationship of the asarones as well as other propenylbenzene phenolethers, many of which are constituents of volatile oils, has been well established1,2,3. Although the Auwers-Skita rule is not strictly applicable in this series of compounds it has been utilized3 in an attempt to elucidate the actual stereochemical configuration in this series4. More recently naves et al., utilizing infrared spectroscopy, have shown that one of the isomer pair of each of isosafrole5, anethol6, isoeugenol, and isoeugenolmethylether7 possesses a characteristic absorption band in a region at 963-967 cm-1, which is in close proximity to the absorption band of trans-ethylenic compounds. The other isomer does not absorb in the same region. The present report is concerned with the stereochemical configuration of asarone and β-asarone as determined by infrared spectroscopy and the chromatographic behavior of the asarones and other propenylphenolethers.
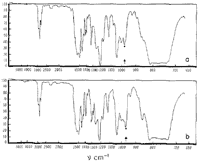
From Fig. 1 it may be seen that asarone absorbs at 964 cm-1 in a manner which is characteristic of trans-propenylbenzenes and that ß-asarone exhibits no absorption in this region. It may therefore be concluded5 that ß-asarone (I) possesses a cis- and asarone (II) a trans-ethylenic side chain.
Table 1
GLC retention time of certain propenylphenolethers
Retention Time (min) |
|||
| Compounds | Ucon polar | Reoplex 400 | Asphalt |
| Isosafrole | 22.5 |
13.0 |
15.0 |
| trans-lsosafrole | 26.5 |
17.0 |
19.0 |
| cis-Isoeugenolmethylether | 30.5 |
20.5 |
20.0 |
| trans-Isoeugenolmethylether | 39.5 |
24.5 |
25.5 |
| cis-Eugenol | 46.0 |
27.5 |
20.5 |
| trans-Isoeugenol | 58.5 |
34.5 |
26.0 |
| β-Asarone | 60.0 |
38.5 |
34.5 |
| Asarone | 82.0 |
57.0 |
50.5 |
Note:
Column 30'x1/4" Liquid phases on Chromosorb P
(35-80 mesh) in a 1:3 weight ratio. Temp: 200°C ±2°C.
Outlet flow: 70 ml of helium per minute.
It was also found that the isomeric pairs of propenylphenolethers could be separated on each of the liquid-gas chromatographic columns used. The best separation can be achieved with a Ucon polar column where the mixture of cis-trans-isosafrole, cis-transisoeugenolmethylether, cis-trans-isoeugenol, and cis-trans-asarone (β-asarone and asarone) can be separated (Fig. 2). It will be observed that in each instance the cis isomer had a lower retention time than the trans (Table 1). There is at present no evidence to indicate that the higher retention times of the trails isomers were clue directly to their configuration and not to their higher boiling points. It would appear, however, that in the propenylphenolethers studied, the trans isomers should exhibit longer retention times than the cis isomers. It is suggestive that this relationship would be applicable independent of the relative polarity of the chromatographic column. This method may allow for the simultaneous separation and the determination of the quantity and geometrical configuration of each individual cis, trans isomer in this series. Subsequent to the presentation of this data, Stahl and Trennheuser8 reported oil the liquid-gas chromatographic separation of the same propenylphenolethers.
Experimental
Asarone and β-asarone were isolated from the volatile oil of Indian Acorus calamus L. (Fritzsche Bros. Inc.) by liquid-gas Chromatography, using a "Ucon" polar column. The samples emerging from the column were collected in acetone and cooled in an acetone-dry ice bath. After the evaporation of the acetone, asarone and ß-asarone were purified and identified as described earlier1.
Infrared Spectra
The spectra of asarone and β-asarone (1.5% w/v in carbon tetrachloride) were obtained using a Beckman IR-4 recording spectrophotometer.
Liquid-Gas Chromatography
An Aerograph A 100-C liquid-gas chromatography unit was utilized. The column temperature was maintained at 200°C ±2°C. Dried helium was used as carrier gas with a flow rate of 70 ml per minute measured at the outlet of the apparatus. The sample (3-5 µL) was injected into a Column with a 10 µL Hamilton fixed needle syringe. In the Case of asarone the sample was dissolved in acetone. The solid support used in the column was Chromosorb P, 35-80 mesh. The liquid phase (6 g) was applied to the solid support in the ratio of 3 to 1 by weight dissolved in a solvent. In the case of Ucon polar (monoether of a random co-polymer of ethylene oxide and propylene oxide, Wilkins Instrument & Research Inc.) and Reoplex 400 the solvent was acetone (100 ml) and in the case of asphalt the solvent was petroleum ether (100 ml, bp 60-80°C). The solution was added to the solid support (18 g) and shaken for 3 hours. The solvent was removed in vacuo at 30°C. After it was dried in a vacuum desiccator the material was packed into a 10'x1/4" (O.D.) copper column with constant tapping.