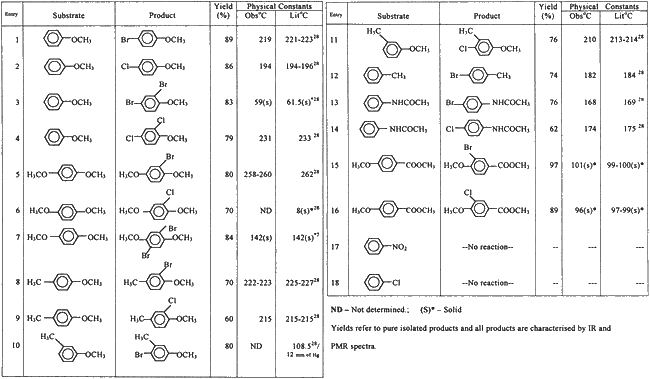
Abstract
A simple and practical halogenation of activated arenes using a reagent combination of potassium halide and Oxone? in water-acetonitrile medium is presented. The halogenated products were obtained with good yields and in high purity.
Introduction
Halogenated aromatic compounds constitute an important class of intermediates due to their easy conversion to other functionalities by simple chemical transformations. Halogenated aromatics, especially brominated ones, are useful not only as precursors to organometallic reagents1 but also are of great importance in the pharmaceutical2, pesticide and agrochemical3 industries.
The direct halogenation with molecular halogen4 has come under increasing scrutiny due to its potential impact on the environment and this suffers from the wastage of halogen as hydrohalous acid. This could be overcome by recycling the same for halogenation in presence of oxidising agents like H2O2,5 tert-butyl-hypohalite,6 tert-butylhydroperoxide7 and sodium perborate.8 The hydrohalous acids are highly corrosive and harmful, as molecular halogen, during large scale preparations, hence the development of new methods of halogenation without using molecular halogen or hydrohalous acid are necessary. The bromination of arenes using organic ammonium tribromides9 (OATB), KBrO3,10 NBS11 over montmorillonite K-10 clay and hexamethylene-tetramine tribromide12 have been reported. However, to the best of our knowledge the chlorination of arenes using similar reagents has not been reported, although, the chlorination and bromination of arenes have been achieved by alkali metal halides in combination of oxidants like NaClO213 and H2O214.
Scheme 1
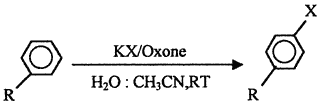
R = Activating groups, (i.e. -OMe, -Me, -NHAc etc.)
Oxone? is a stable ternary composite of KHSO5, KHSO4 and K2SO4 in 2:1:1 molar proportions. Its utility as an oxidant has been demonstrated in the oxidation of sulphides to sulphoxides and sulphones,15 selenides to selenones,16 s-alcohols to ketones,17 acetals to esters,18 nitriles to aldehydes,19 amines to nitro-compounds,20 hydrazides to diacylhydrazines21 and of benzoins to diketones.22 It has also been used for the generation of carbonyl compounds from oximes,23 hydrazones, semicarbazones, for oxidative Nef reaction24 and in the cleavage of tert-butyl dimethyl silyl ethers.25 The literature reports also revealed the use of metal halide and Oxone? for halogenation of pyrimidines,26 α,β-enones and alkenes27 with alkali halides but so far, it has not been used for halogenation of arenes.
We wish to report herein the halogenation of activated arenes with potassium halide using Oxone? as an oxidant, in water-acetonitrile medium (Scheme 1). The results of halogenation of arenes are summarised in Table 1.
Oxone? though soluble in water, is insoluble in most of the common organic solvents. Hence halogenations were performed in a solvent mixture of water-acetonitrile (3:1, v/v) in which both, the substrates and Oxone?, are freely soluble. This constitutes a single phase and halogenation proceeds smoothly at room temperature within 2?3 h. The use of one equivalent of each, Oxone? and alkali metal halide, yields monohalogenated product while two equivalents of same furnishes dihalogenated products. The halogenation studies with various arenes revealed that, only activated arenes undergo halogenation smoothly to yield para halogenated products and if para position is blocked, ortho halogenation results. In case of the deactivated arenes, halogenation was not observed even after prolonged reaction time (10 h). Further, when halogenation of toluene was carried out, nuclear halogenation took place instead of side chain halogenation which is indicative of electrophilic substitution mechanism rather than the radical pathway.
In conclusion, the present procedure is simple, convenient and less expensive. In addition the reagent used is non toxic and easy to handle.
Experimental
Oxone? (Lancaster), KX (E. Merck), Acetanilide, Toluene, p-Hydroxy benzoic acid and Cresols (SD Fine Chem) were used. Cresyl methyl ethers, 1,4-Dimethoxybenzene and 4-Methoxymethylbenzoate were prepared from the corresponding hydroxy derivatives by standard methods reported in the literature31.
Typical Procedure for Bromination of 1,4-Dimethoxybenzene
Monobromination: To a stirred solution of Oxone? (2.5 mmol) in 30 ml water: acetonitrile (3:1, v/v) was added the solution of 1,4-dimethoxybenzene (2.5 mmol) in acetonitrile (3 ml). Potassium bromide (2.5 mmol) was added and stirring was continued at room temperature till the completion of reaction (TLC). Water (20 ml) was then added and the reaction mixture was extracted with ether (2?30 ml). The ether extract was washed with water, dried over Na2SO4 and ether removed to yield 2-bromo-1,4-dimethoxy benzene. It was chromatographed over silica gel and elution with light petroleum afforded pure compound, (Table 1A, 1B, Entry 5) (0.44 g, 80%), b.p. 258?260?C (lit.28 262?C).
PMR (CDCl3): δ 3.76 (3H, s, Ar-OCH3), 3.83 (3H, s, OCH3 ortho to-Br), 6.83 (2H, d, Ar-H ortho to?OCH3) and 7.11 (1H, bs, ArH ortho to?Br).
Dibromination: It was carried out as above except the quantities of potassium bromide and Oxone? were doubled. The workup afforded 2,5-dibromo-1,4-dimethoxybenzene, (Table 1A , 1B , entry 7) which was purified by crystallisation (CHCl3 + light petroleum), yield (0.640 g, 84%), m.p. 142?C (Lit.7 142?C).
PMR (CDCl3): δ 3.85 (6H, s, 2 ? Ar OCH3), 7.11 (2H, s, ArH ortho to ?OCH3).