Reduction of Phenylalanine to Amphetamine[ Back to the Chemistry Archive ] Synthetic outline:
This is what they call a enantiomerically pure reduction of amino acids. It means that if you start with D-phenylalanine, that you get the good stuff; dexedrine. You will need a well equipped lab, do not try this in your kitchen: the reactions are carried out in flame-dried flasks under a dry nitrogen atmosphere. I think that the reaction where they substitute the alcohol for the iodide, is the Mitsunobu reaction, but it seems that they have used imidazole instead of diethyl azodicarboxylate (DEAD) to activate the triphenylphosphine toward nucleophilic attack by the alcohol. ProcedureStep 1: D-Phenylalaninol 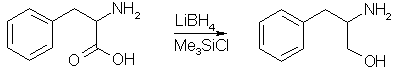
To a cold solution of LiBH4 (1.32g, 60.54 mmol) in THF (30 ml, freshly distilled from LiAlH4) wass added trimethylsilyl chloride (15.36 ml, 121.07 mmol). The ice/water bath was removed and the mixture was allowed to stir at room temperature for 15 min. The mixture was recooled to 0°C and D-phenylalanine (5g, 30.27 mmol) was added. The ice/water bath was removed, and the reaction mixture was stirred overnight. The mixture was again cooled to 0°C and MeOH (45 ml) was added dropwise, followed by 2.5 M aqueous NaOH (25 ml). This mixture was evaporated in vacuo, and the residue extracted 5x with chloroform. The combined extracts were dried (Na2SO4), filtered, and evaporated in vacuo to leave 4.55 g (99%) of the product as a white crystalline solid, mp 88-90°C. Step 2: N-t-Boc-D-phenylalaninol 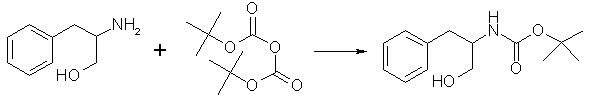
To a stirred, chilled (0°C) solution of D-phenylalaninol (5 g, 33.1 mmol) in 85 ml of chloroform was added solid di-tert-butyl dicarbonate (7.22g, 33.1 mmol). The solution was stirred at 0°C for 30 min and then stirred at room temperature overnight. The solution was washed with 20% phosphoric acid, a saturated NaHCO3 solution, and a saturated NaCl solution, then dried (Na2CO3) and evaporated to dryness under reduced pressure. The resulting solid was recrystallized from a hot hexane/ethyl acetate mixture to afford 7.48g (95%) of the product as white fibrous crystals, mp 96°C. Step 3: tert-Butyl (1R)-1-Benzyl-2-iodoethyl Carbamate 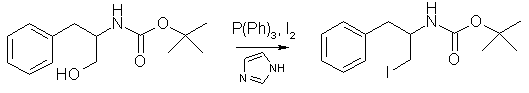
To a stirred, chilled (0°C) suspension of 2.92 g of polymer-supported triphenylphosphine (8.75 mmol) in dry DCM (35 ml) was added 2.22 g of iodine (8.75 mmol), followed by 0.65 g of imidazole (9.55 mmol). The mixture was allowed to warm to ambient temperature, and after 30 min a solution of 1.26 g (3.98 mmol) of N-t-Boc-D-phenylalaninol in DCM (15 ml) was added dropwise. The mixture was then heated at reflux for 2 hours. The cooled mixture was then filtered and the solution waswashed with dilute aqueous Na2S2O3 and water, dried (Na2SO4), and evaporated to a white crystalline solid. Passing the residue through a short silica gel column (3:2, EtOAc:hexane) yielded the pure product which was recrystallized from hot hexane to obtain 1.09 g of product (88 %) as white crystals, mp 121-122°C. Step 4: tert-Butyl (1S)-1-Methyl-2-phenylethyl carbamate 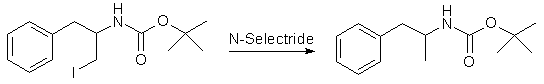
A solution of 1.00g (2.77 mmol) of the iodo compound in anhydrous THF (20 ml) was stirred at -15°C as 3.04 ml (3.04 mmol) of a 1.0 M solution of N-Selectride (in THF) was added dropwise via syringe. The mixture was allowed to warm to 5°C over 1.5h. Reaction progress was monitored by TLC (4:1 hexane:EtOAc). The solution was cooled to 0°C, and the reaction was quenched by the slow addition of 1.3 ml of water. This was followed by the dropwise addition of a solution made by combining 15 ml of H2O, 1.0 g of K2CO3, and 2.6 ml of 30% H2O2. The reaction mixture was stirred at ambient temperature for 1h. The THF was evaporated under reduced pressure, and the product was extracted from the residue with 3 portions of DCM. The organic extracts were dried (Na2SO4) and the solvent evaporated to yield a white solid. Passing this material through a short silica column (4:1 hexane:EtOAc) yields the product (0.61g, 94%) as a white crystalline solid. Step 5: (1S)-1-Methyl-2-phenylethylamine HCl (Dexedrine, d-amphetamine) 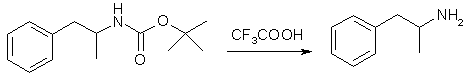
To a stirred, cooled (0°C) solution of tert-Butyl (1S)-1-Methyl-2-phenylethyl Carbamate (2.59 g, 11.0 mmol) in DCM (20 ml) was added trifluoroacetic acid (5 ml). The solution was stirred at ambient temperature for 18 h. The volatile components were reduced under reduced pressure, and the residure was treated with water (10 ml), chloroform (15 ml) and a 50% NaOH solution (2 ml). The mixture was shaken, and the layers were separated. The aquous layer was extracted five times with chloroform and the combined organic extracts were dried over Na2SO4 and filtered. To this was added 6 ml of a 1.0 M HCl solution (in Et2O) and the solvents were removed to yield a yellow solid. This was recrystallized in hot hexane/acetone to yield the product as white, needle-shaped crystals, 1.34 g (91%), [alpha]25D = 9.21 (c 9.56, MeOH). References[1] Journal of Organic Chemistry 65(16), 5037 (2000) |