On the basis of the number of clandestine laboratories seized by the Drug Enforcement Administration (DEA), the use of clandestinely synthesized drugs would appear to be increasing. The number of laboratories closed by the DEA has increased from 184 laboratories in fiscal year 1981 to 647 laboratories in fiscal year 1987. Although many risks are associated with the abuse of drugs, purity is a major danger associated with these clandestinely prepared drugs (Soine 1986). Compounds which can affect drug purity can be classified as diluents, adulterants, impurities of manufacture and impurities of origin. The presence of diluents and adulterants is highly variable, however, this is not true for the impurities associated with the synthetic process. The impurities of manufacture and origin are usually present and will vary primarily in concentration. Therefore, it is not surprising that these impurities may contribute to the pharmacological or toxicological effects associated with clandestinely synthesized drugs.
This report updates information that was published in a prior review on this subject (Soine 1986). The drugs discussed are limited to the stimulants, amphetamines and cocaine. The synthetic method for each drug is discussed, followed by the identification and occurrence of synthetic impurities and their associated pharmacology/toxicology.
Amphetamines
Figure 1
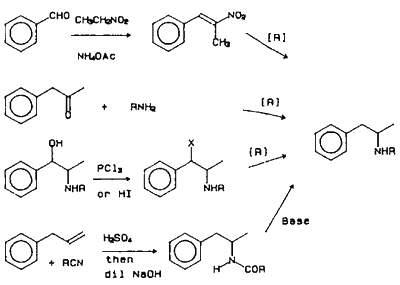
There are numerous methods reported for the synthesis of amphetamine (Frank 1983), as shown in Figure 1. The major methods utilized by the clandestine chemists use starting materials that can be easily purchased and/or synthesized. The major amphetamine synthesized in the United States is methamphetamine, whereas in Europe, it appears to be amphetamine. An important precursor for the synthesis of both amphetamine and methamphetamine is phenyl-2-propanone (P2P). Four basic synthetic routes are used for the synthesis of P2P (Frank 1983). The most frequently used method starts with phenylacetic acid, acetic anhydride and sodium acetate or pyridine. It is this reason that sales of large quantities of phenylacetic acid (1 kg) are monitored by the DEA. The most commonly observed by-product present in P2P prepared by this method is dibenzylketone (Herbst and Manske 1943). Dibenzylketone will produce a synthetic impurity via the same reductive amination reactions as P2P to give 2-(phenylmethyl)-phenethylamine (or the N-methyl homolog in the case of methamphetamine). The methods using allylbenzene or β-methylstyrene (Dal Cason et al., 1984) are analogous to the methods used clandestinely for the synthesis of 3,4-methylenedioxyphenyl-2-propanone (to be used in the synthesis of 3,4-methylenedioxyamphetamines) from safrole or isosafrole (Ellern 1985, Hanson 1988). Impurities present in P2P using allylbenzene or β-methylstyrene have not been reported.
The Leuckart method is still frequently encountered as a method for synthesis of both amphetamine and methamphetamine. Until 1981, the main impurity present in amphetamine clandestinely prepared in the Netherlands was 4-methyl-5-phenylpyrimidine (Huizer et al., 1985). After 1981 di-(β-phenylisopropyl)-amines, DPIA, (or the N-formyl derivative, or both) were the main impurities observed in amphetamine in both the Netherlands and in Norway (Lambrechts et al., 1986). It was hypothesized that the change in impurity pattern was due to the addition of formic acid during the P2P and formamide condensation step. It should be noted that DPIA isomers have two chiral centers, therefore both a diastereomeric pair and a meso stereoisomer are usually present in illicit amphetamines prepared via the Leuckart method.
The most popular method for the synthesis of methamphetamine is via reductive amination using P2P, methylamine, aluminum foil, mercuric chloride (catalytic amount) diethyl ether and isopropanol (Frank 1983). This reaction goes in very high yields and a very pure product can be obtained. It has been observed that samples of illicit methamphetamine from numerous clandestine laboratories have been found to contain mercury ranging from trace levels to 1300 ppm mercury (Davidson 1983). Additional basic contaminants have not been reported for this synthetic route.
Figure 2
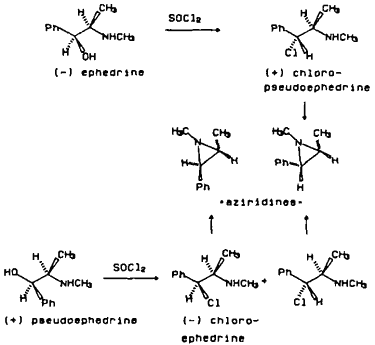
The second most frequently encountered synthesis of methamphetamine is from ephedrines by conversion to the chloroephedrines followed by reduction (Allen and Kiser 1987, Cantrell et al., 1988). (-)-Ephedrine or (+)-pseudoephedrine is converted to (+)-chloropseudoephedrine or (-)-chloroephedrine, respectively, using SOCl2, PCl3, PCl5 or POCl3. The reductive dehalogenation to methamphetamine is accomplished using H2/catalyst, HI or HI and red phosphorous (DEA Dec. 1986). The synthetic impurities present in samples using this method are (+)-chloropseudoephedrine or (-)-chloroephedrine which also cyclize to form cis- or trans-1,2-dimethyl-3-phenylaziridines, (Figure 2). All of these impurities have been detected in varying levels in clandestinely prepared (+)-methamphetamine. When synthesizing methamphetamine using this approach or amphetamine from phenylpropanolamine, retention of configuration at the amine carbon occurs. The enantiomeric composition of illicit amphetamine or methamphetamine is rarely reported.
Additional amphetamine analogs identified in the illicit drug market since January 1984 are N,N-dimethylamphetamine (synthesized from N-methylephedrine using HI and red phosphorous) (Tackett et al. 1988, Bond 1988), N,N-dimethylphenethylamine (Clark 1986), N-ethylamphetamine (DEA Dec. 1985), 4-methoxyamphetamine (DEA Aug. 1985), 4-methoxymethamphetamine (DEA July 1987), N-(2-hydroxyethyl)-amphetamine (Heagy and Allen 1987). N-(2-cyanoethyl)-amphetamine (DEA Aug. 1987), and 4,5-dihydro-4-methyl-5-phenyl-2-oxazoline (U4Euh, prepared by the condensation of phenylpropanolamine with cyanogen bromide) (Inaba and Brewer 1987, Davis and Brewster 1988). The impurities from manufacturing have not been reported for these compounds.
The pharmacology reported for the synthetic contaminants in amphetamines is very limited except for 2-(phenylmethyl)-phenethylamine (Soine 1986). In a recent study the intraperitoneal LD50 and CD50 (convulsive dose 50%) of 2-(phenylmethyl)-phenethylamine and N-methyl-2-(phenylmethyl)-phenethylamine in mice was evaluated in relation to amphetamine and methamphetamine (Noggle et al. 1985). The LD50 values for both contaminants were comparable to that of both amphetamine and methamphetamine, however, the contaminants exhibited different non-lethal symptoms compared with animals given the corresponding amphetamines. In doses well below the LD50 the contaminants exhibited marked convulsive episodes characterized by clonic and tonic seizures which suggest greater CNS stimulation by the contaminants at the brain stem and cord levels. These symptoms were seen for the amphetamines only at doses at or near the amphetamine LD50 doses. The information concerning the pharmacology of the other synthetic impurities is minimal (Soine 1986).
Cocaine
Cocaine is obtained from the leaves of coca plants belonging to the genus Erythroxylum (family Erythroxylaceae) with the major species used for the legal and illegal production of cocaine being E. coca Lam. var. coca ("Bolivian" or "Huanuco coca") (Novak et al., 1984). In general the primary method for preparing illicit cocaine in the coca-producing area involves crushing and soaking the leaves in a Na2CO3/kerosene mixture. Upon filtration, the kerosene filtrate is extracted with dilute H2SO4. The acid extract is made basic and the solid precipitate obtained is referred to as coca paste or cocaine sulfate base. Coca paste varies in cocaine content from 30-90% cocaine. This coca paste is further refined in cocaine "crystal" laboratories. During this process the coca paste is dissolved in a dilute H2SO4 solution, the insolubles are filtered off and the acid solution is made basic with ammonium hydroxide or an ammonium hydroxide/NaHCO3 buffer. The cocaine base, which is relatively pure (90-95%), precipitates. The cocaine base is dissolved in acetone/ether to which concentrated HCl is added from which very pure cocaine hydrochloride precipitates. Depending on the skill of the operator and the stage at which the sample is obtained, numerous other alkaloids, in addition to cocaine, will be present. The alkaloids containing a tropane nucleus include benzoylecgonine, methylecgonine, ecgonine, cis- and trans-cinnamoyl cocaine, α- and β-truxillines, and tropacaine. Due to crystallization behavior similar to cocaine, the cinnamoylcocaines are frequently detected, and are usually present in very low concentrations (less than 5%) (Soine 1986).
Due to the crude processing facilities that are used, residual solvents are usually present in illicit cocaine. Because these solvents may be indicative of solvent requirement or if samples are being obtained from a common source the DEA has carried out studies characterizing the volatile components present in cocaine HCl. Prior to 1981 all cocaine samples contained trace levels of acetone and ether. Recent studies indicate that acetone/ether is still preferred for cocaine processing (49% of the samples). However, a decreasing availability of diethyl ether in South America appears to have forced the usage of other solvents such as acetone, methylethylketone (Churchill 1985) and benzene (Kiser 1986). Volatile compounds that are also associated with illicit cocaine samples are the hydrocarbons present in kerosene (alkylbenzenes and hydrocarbons), contaminants of acetone (mesityl oxide and diacetone alcohol), and trans-esterification products from cocaine (methyl benzoate, methyl cinnamate and dimethyltruxillate) (Pettit 1986).
Figure 3
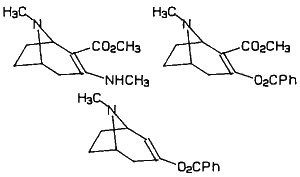
Numerous methods for the total synthesis of cocaine have been reported in the scientific literature and many of these methods have been attempted by the clandestine chemists (Cooper and Allen 1984). When cocaine is obtained by total synthesis it is referred to as "synthetic" cocaine. To date all seizures of operating clandestine laboratories have utilized routes through the common intermediate, 2-carbomethoxytropinone. The most commonly observed route is that described in "head shop" publications which contain reprints of the work by Preobazhenski (1936). The least commonly encountered method, although it is usually used by the most experienced chemists, is the method described by Findlay (1957). "Synthetic" cocaine is identified by the presence of the diastereomers of cocaine (pseudococaine, allococaine or allopseudococaine) or by the d enantiomer of cocaine. Additional contaminants common to most of the synthetic routes are shown in Figure 3. Contaminants that are present in both synthetic and natural cocaines are benzoyl pseudotropine and benzoyltropine.
The biological activity associated with the naturally occurring alkaloids of cocaine (other than cocaine and nicotine) have been comprehensively reviewed up to 1984 (Novak et al., 1984). Based on this review no recent reports are available concerning the pharmacological activity of the tropane alkaloids methylecgonidine, pseudotropine, α- and β-truxilline, and the pyrrolidine alkaloids hygrine, hygroline and dihydrocuscohygrine. Cinnamoylcocaine had previously been reported to have no pharmacological activity (Woker 1953). Recent studies have shown that cinnamoylcocaine(s) suppress the primary humoral (PFC) immune response of mice following oral administration (Watson et al., 1983). The presence of a potential Michael adduct in cinnamoylcocaine could be associated with this response, however, this is unlikely since this effect was less than that observed for cocaine at the same dose. In in vitro studies (-)-trans-cinnamoylcocaine was one order of magnitude less potent than cocaine in binding to the central high-affinity [3H]-cocaine binding site, but was comparable to cocaine in binding to the peripheral (liver) high-affinity cocaine binding site (Calligaro and Eldefrawi 1987). The α- and β-truxillines are reported to have no anesthetic action although they were originally reported as strong heart toxins (Liebermann 1888). The activity associated with the remaining ecgonine alkaloids suggest that these compounds do not contribute significantly to the CNS effects associated with cocaine (Novak et al. 1984).
It is hoped that this review will bring attention to some variables that can influence the pharmacological effects of clandestinely prepared drugs. Illicit drugs are usually impure and will predictably contain certain synthetic contaminants. Unfortunately, very little is known concerning the acute and chronic effects of these compounds.