Oxidation of olefin-type substrates with KMnO4 yields mostly dihydroxylated or hydroxy oxo products in alkaline or neutral media, and the double bond is cleaved to the corresponding acids in acidic solutions1-9. A series of studies on the mechanism and intermediates of such oxidations in aqueous solution have revealed the transient formation of aldehydes, which, however, undergo further oxidation by manganese(II) and/or manganese(IV) intermediates8. No attempt was made at utilizing the cleavage reaction on a preparative scale, although the possibility of quenching the manganese intermediates for saving the aldehydes had been pointed out8b-e. Under phase-transfer conditions, isolated examples of olefin to aldehyde conversion have been reported2,7. There is, however, no convenient procedure for double bond cleavage to aldehydes in aqueous organic mixtures which would not require special additivies (phase-transfer agent) or techniques (chemical quenching). We now report a simple method in THF/water, in which the solvent plays the role of a quenching reagent, permitting good yields of the aldehydes.
Table I.
Yield of Aldehyde R1CHO
R1 |
R2 |
R3 |
Yield |
R4 | H | Ph |
78.7% |
Ph | CO2Et | CO2Et |
37.5% |
Ph | H | Ph |
70.5% |
i-Pr | CO2Et | CO2Et |
14.2% |
CO2Et |
H | CO2Et |
50.5% |
CO2Et | CO2Et |
H |
47.9% |
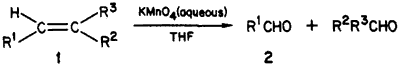
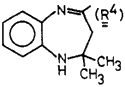
In a mechanistic study on 1,5-benzodiazepines, we needed compound R4CHO for identifying a reaction intermediate. Attempts at converting the 4-methyl of R4CH3 to a formyl group via oxidation or dihalogenation/hydrolysis were unsuccessful. We have found however that treatment of a dilute THF solution of 1 (with R1=R4, R2=H, and R3=Ph) by a concentrated aqueous solution of KMnO4 afforded the desired aldehyde R4CHO and benzaldehyde in 80% yield. The lack of extensive overoxidation is surprising since in the overwhelming majority of reported cases cleavage leads to the corresponding acids. The testing of this procedure on some other olefinic compounds indicates that it may be useful in the synthesis of a variety of aldehydes. Examples are listed in Table I. Apparently, activation of the double bond by conjugation increases, whereas adjacent bulky groups decrease the yield.
Experimental
4-Formyl-2,2-dimethyl-1H-1,5-benzodiazepine (2, R1=R4)
To a solution of 10 g (0.036 mol) of 1 in 300 cm3 of THF was added 10 g (0.063 mol) of KMnO4 dissolved in 100 cm3 of water, over a period of 3.5 h in small portions. The reaction mixture was allowed to warm up to 40 °C. After the addition was finished, the brown precipitate was filtered, and the filtrate was concentrated and extracted with diethyl ether. After drying, the organic phase was concentrated and the resulting oil crystallized from diisopropyl ether: 7 g (78.7%); mp 102-104°C.
The above procedure can be used to synthesize all of the other aldehydes listed in Table I. Solubility of the starting material determines the THF/H2O ratio. In case of low solubility, a few preliminary tests should be made to determine the minimum amount of THF.
Attention: Usage of neat THF or addition of solid KMnO4 may lead to an explosion and therefore must be avoided.
Yields were determined by GC analysis (5% QF-1 column) of the Et2O phase after suitable dilution.
4-(2-Phenylvinyl)-2,2-dimethyl-1H-1,5-benzodiazepine (1, R1=R4, R2=H, R3=Ph)
A solution of 10 g (0.053 mol) of 2,2,4-trimethyl-1H-1,5-benzodiazepine, 5.63 g (0.053 mol) of benzaldehyde, and 0.5 g of ammonium acetate in 100 cm3 benzene was refluxed for 4 h and then concentrated. The resulting oil is crystallized from diisopropyl ether: 12.1 g (82.4%); mp 134-136°C.