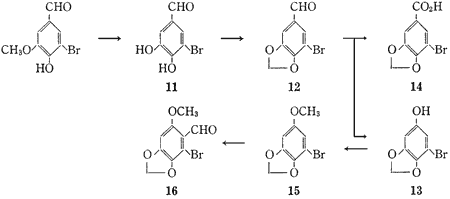
5-Bromoprotocatechualdehyde (11) was obtained from 5-bromovanillin1 quantitatively by applying the method which Lange2 reported for the cleavage of various alkyl o-hydroxyphenyl ethers. This bromoaldehyde (11) was treated with methylene iodide in dimethylsulfoxide (DMSO) in the presence of anhydrous potassium carbonate to give 5-bromopiperonal (12) in 38.8% yield. The Baeyer-Villiger oxidation of 5-bromopiperonal (12) with performic acid gave 5-bromosesamol (13), mp 102-104°C, in 78.8% yield, accompanied by a small amount (0.6%) of 5-bromopiperonylic acid (14). Methylation of 5-bromosesamol (13) in the usual way gave 1-bromo-5-methoxy-2,3-methylenedioxybenzene (15), mp 49-51°C, in 96.5% yield. Treatment of this methoxybenzene (15) with phosphorus oxychloride and dimethylformamide (DMF) gave 2-bromo-6-methoxy-3,4-methylenedioxybenzaldehyde (16), mp 208-209°C, as the sole product in 91.5% yield.
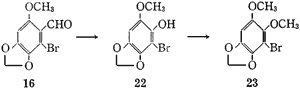
The Baeyer-Villiger oxidation of the aldehyde (16) with performic acid afforded 2-bromo-6-methoxy- 3,4-methylenedioxyphenol (22), mp 128-129°C, in 72.9% yield, which was converted to 1-bromo-2,3-dimethoxy- 5,6-methylenedioxybenzene (23), mp 78-79°C, by methylation with diazomethane in 97.9% yield or with dimethyl sulfate and 50% sodium hydroxide solution in methanol in 96.3% yield.
Experimental
5-Bromopiperonal (12)
A mixed solution of bromoprotocatechualdehyde3 (11) (20.0 g; mp 225-230°C), CH2I2 (49.7 g), and anhydrous K2CO3 (35.5 g) in DMSO (61.5 ml) was heated at 60°C for 32 hr in a nitrogen stream, and poured into a large quantity of water. The precipitate was filtered off and purified by column chromatography on Al2O3 (Brockmann) using CHCl3 as solvent to give colourless needles (14.5 g), mp 124-126°C (lit.4 mp 120°C; lit.5 mp 124-125°C).
5-Bromosesamol (13)
To 85% HCOOH (80 ml) was added 35% H2O2 (24 g) under ice cooling. The mixture was stirred at room temperature for 1 hr. A solution of 5-bromopiperonal (12) (35.32 g) in 98% HCOOH (740 ml) was added to the solution of performic acid below 10°C and stirred at 0°C for 4 hr. After decomposition of the excess peracid by addition of Na2SO3 (35 g), the reaction mixture was poured into ice-water and extracted with ether. The ether solution was washed with saturated NaHCO3 aq., added to 5% NaOH aq. (500 ml) with vigorous stirring and stirred at room temperature for 30 min. The aqueous layer was made acidic with 10% HCl aq. and extracted with ether. The ethereal solution was dried over MgSO4 and evaporated to dryness in vacuo. Recrystallization of the residue from benzene-hexane gave colourless needles (26.4 g), mp 102-104°C.
Acidification of the saturated NaHCO3 solution gave 5-bromopiperonylic acid (14) (0.28 g), mp 250-252°C (lit.4 mp 253°C).
1-Bromo-5-methoxy-2,3-methylenedioxybenzene (15)
To a solution of 5-bromosesamol (13) (6.00 g) in MeOH (16 ml) containing dimethyl sulfate (3.15 ml) was added 2 N NaOH aq. (52 ml) at room temperature. The mixture was kept at room temperature and addition of the same amounts of dimethyl sulfate and 2 N NaOH aq. was repeated three more times, monitoring by TLC. Finally, after further addition of 2 N NaOH aq. (50 ml), the mixture was refluxed for 30 min, cooled and extracted with ether. The ethereal solution was dried over MgSO4 and evaporated to dryness in vacuo. Distillation of the residue at 109°C (1.5 mmHg) gave an oily substance (6.17 g) which crystallized when kept in a refrigerator. Recrystallization of a portion of the crystalline from hexane gave colorless needles, mp 49-51°C.
2-Bromo-6-methoxy-3,4-methylenedioxybenzaldehyde (16)
Phosphorus oxychloride (15 ml) was added over 1 hr to a solution of the methoxybenzene (15) (25.5g) in dry DMF (25 ml) under ice cooling. After addition, the mixture was heated at 60°C for 3 hr, poured into ice-water, made alkaline with 5% NaOH aq. and filtered. Recrystallization of the precipitate from EtOH or CHCl3/hexane gave colourless needles (26.2 g), mp 208-209°C.
2-Bromo-6-methoxy-3,4-methylenedioxyphenol (22)
After addition of 35% H2O2 (5.7 g) to 85% HCOOH (19.4 ml) at 0°C, the mixed solution was stirred at room temperature for 1 hr, and cooled again to 0°C. A solution of 2-bromo-6-methoxy-3,4-methylenedioxybenzaldehyde (16) (7.7 g) in 98% HCOOH (310 ml) was gradually added to the cooled performic acid solution at 0-5°C and then stirred at 0-5°C for 5 hr. After decomposition of the excess peracid by addition of Na2SO3 (7.7 g), the reaction mixture was diluted with an equal volume of water and extracted with ether. The ethereal solution was washed with saturated NaCl aq. and then saturated NaHCO3 aq. After addition of a 5% NaOH aq. to the ethereal solution, the two layers were vigorously stirred at room temperature for 1.5 hr. The ethereal solution was separated from the 5% NaOH aq. and washed with 5% NaOH aq. The original 5% NaOH aq. and washings were combined, made acidic with 17% HCl aq., and extracted with ether. The ethereal solution was dried over MgSO4 and evaporated to give colourless prisms (5.35 g), mp 128-129°C, which were recrystallized from EtOH. The ethereal solution separated from the 5% NaOH aq. was dried over anhydrous K2CO3 and evaporated to give unchanged starting aldehyde (16) (250 mg).
1-Bromo-2,3-dimethoxy-5,6-methylenedioxybenzene (23)
- Using Diazomethane:
A solution of CH2N2 (nitrosomethyl urea: 600 mg) in ether (5 ml) was added to a solution of the phenol (22) (164 mg) in MeOH (1 ml). The mixed solution was allowed to stand at room temperature for 30 min and evaporated to give colourless needles (175 mg), mp 78-79°C, which were recrystallized from benzene-hexane. - Using Dimethyl Sulfate:
To a solution of the phenol (22) (9.5 g) in MeOH (50 ml) containing dimethyl sulfate (16 ml) was added 50% NaOH aq. (10 ml). After the reaction mixture was heated under reflux for 1 hr, another 50% NaOH aq. (10 ml) was added. The mixture was allowed to stand at room temperature overnight, poured into a large quantity of water and extracted with ether. The ethereal solution was washed with 5% NaOH aq., dried over anhydrous K2CO3 and evaporated. The residue (9.56g), mp 78-79°C, was identical with the sample prepared by treatment with CH2N2.