Introduction
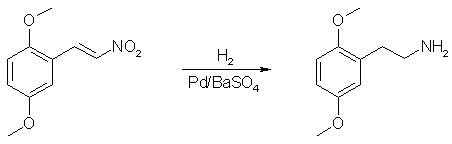
The hydrogenation of 2,5-dimethoxy-β-nitrostyrenes to obtain the corresponding saturated amine has been attempted with various catalysts. Efforts to reproduce this reduction by prior art processes have resulted in poor yields and/or products which were difficult to purify. Use of palladinized barium sulfate, as herein contemplated, provides a simple reduction process with high yields and reaction products which may be simply and readily worked up.
The reduction is preferably performed in a mixture of acetic and sulfuric acids as the reaction medium. Suitable reaction conditions are room temperature and a pressure of 2-3 atm. The reaction is preferably run for a short period after hydrogen uptake ceases.
Conventional palladinized barium sulfate catalysts may be used in this process. A preferred catalyst comprises about 7-8 weight% of palladium. It may be prepared by precipitating barium sulfate in the presence of freshly precipitated palladium.
Experimental
2,5-dimethoxy-β-nitrostyrene (5g) is dissolved in a mixture of glacial acetic acid (125 ml) and concentrated sulfuric acid (19g). Five grams of palladinized barium sulfate are added and hydrogen is passed through the mixture at room temperature. The mixture is shaken continually during the reaction. Ninety percent of the theoretical amount of hydrogen (4 moles) is absorbed in 10 minutes, after which no further hydrogen uptake is noted. Shaking of the mixture is continued for a half hour, after which the mixture is cooled and sufficient 5 N sodium hydroxide is added to neutralize the sulfuric acid. Methanol (250 ml.) is then added to complete precipitation of sodium sulfate. The salt is washed and filtered with methanol and the combined filtrates and mother liquor are evaporated in vacuo. The residue is made strongly alkaline and extracted with 100 ml. of ether. The extract is dried over potassium hydroxide after which the solvent is removed and the residue distilled giving 3g (68%) of 2,5-dimethoxyphenethylamine boiling at 148°C/8mmHg. The product is a pale yellow oil which forms a white hydrochloride melting at 139°C.