The Neuropharmacology of Hallucinogens
a technical overview
v3.1 Aug 2005
edited & published by Erowid
Citation: BilZ0r. "The Neuropharmacology of Hallucinogens: a technical overview".
Erowid.org, v3.1 Aug 2005: psychoactives/pharmacology/pharmacology_article2.shtml.
Please note that this subject is extremely technical and this article requires a background in brain science to fully understand. Some definitions of some key terms are included in the Definitions section of this artice.
Introduction #
What are hallucinogens? #
In common usage, the word "hallucinogen" has become a catchall term for a wide variety of psychoactive substances, including such pharmacologically different chemicals as cannabinoids; NMDA receptor antagonists such as ketamine; 3,4-methylenedioxymethamphetamine; kappa-opioid agonists such as salvinorin A, and lysergic acid diethylamide (LSD). The term hallucinogen is often used in medical and research journals, but is rarely given a precise definition. When used in its most general sense, it is vague, adds confusion to complex neuropharmacological issues, and almost becomes meaningless.
|
What is in a name? #
Hallucinogens produce effects in the mind that can be so intense, that they have led
people to suggest other names for this group, such as 'entheogens' (Ruck et al., 1979)
and 'psychotomimetics' (Clark, 1956)1. Entheogen is derived from
the Greek, en-theos, which means "god/spirit within" and is in reference to the
spiritual and deep effect hallucinogens can have on the mind, while psychotomimetic
means "psychosis mimicking", and refers to the similarities between hallucinogen-induced
states and schizophrenia and other mental illnesses. These two divergent terms
illustrate why the study of hallucinogens is important: an understanding of how
hallucinogens affect thought could help explain not only how mental illness affects the
mind, but also the nature of consciousness itself.
How do hallucinogens work? #
The history of mechanistic hallucinogen research #
Once serotonin's (5-HT) presence was demonstrated in the brain in 1953 (Twarog & Page,
1953) it was not long until the chemical similarity between LSD and 5-HT was noted. In
the same year Gaddum (1953) demonstrated that LSD antagonized the action of 5-HT in
peripheral tissues. Soon afterwards two groups independently proposed the hypothesis
that the hallucinogenic action of LSD was due to its ability to block central 5-HT
receptors (Gaddum & Hameed, 1954; Woolley & Shaw, 1954).
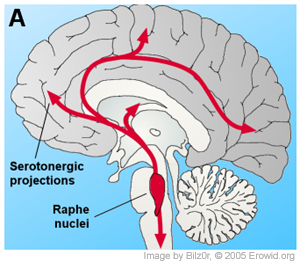
|
It was discovered early in research, that hallucinogens of various types reduced serotonin turnover in the brain and this was mirrored by the fact that systemically applied (injected into the body or given orally, not injected into the brain) hallucinogens inhibited the firing of cells in the dorsal raphe nucleus (Aghajanian et al., 1970; Aghajanian et al., 1968). Furthermore, it was shown that indole hallucinogens, microiontophoretically applied to the dorsal raphe, inhibited the firing of cells there (Aghajanian et al., 1972; Aghajanian & Hailgler, 1975; de Montigny & Aghajanian, 1977). Indeed, the ability for these chemicals to inhibit raphe cell firing seemed a likely hypothesis for their mechanism of action, as these cells are the source of cortical serotonin and hence inhibiting them could cause changes throughout the brain consistent with the broad effects of hallucinogens.
"It is generally agreed that 5-HT2 receptor subtypes are the primary site of action of hallucinogens."
-- Connelly B
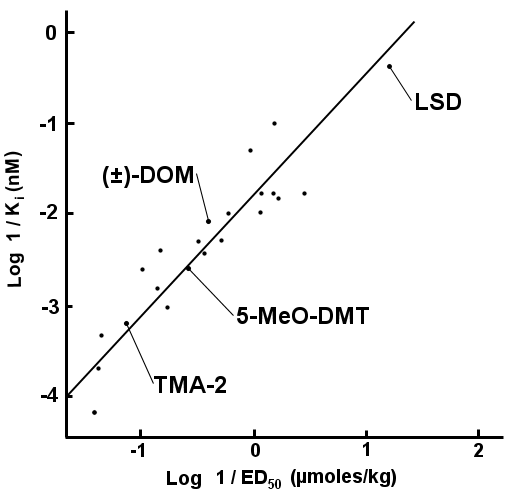
|
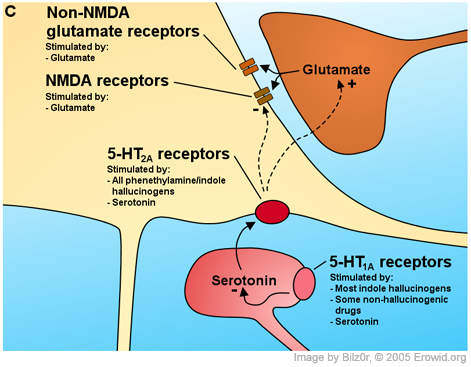
This correlation has been shown several times (Glennon et al., 1986; Glennon et al., 1984b; Sanders-Bush et al., 1988), and along with a large number of agonist/antagonist experiments (reviewed by Nichols, 2004) it is generally agreed that 5-HT2 receptor subtypes are the primary site of action of hallucinogens.
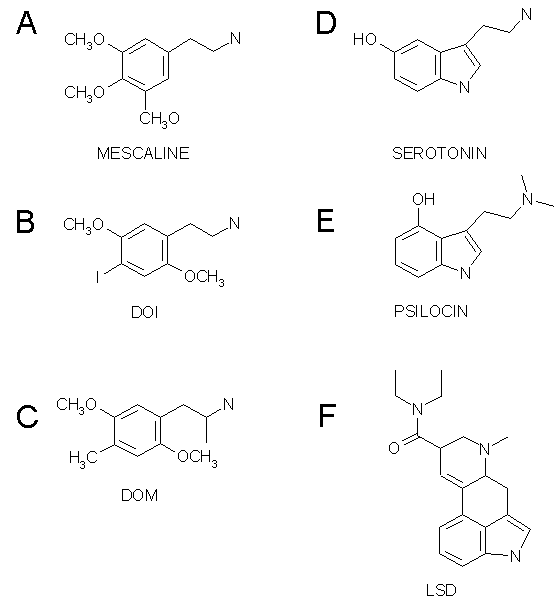
As the documented number of 5-HT receptor subtypes proliferated, the main question became which subtype mediated the hallucinogenic action of hallucinogens. LSD binds potently to the 5-HT1A/1B/1D/1E/1F (Hoyer, 1988; Lovenberg et al., 1993b), to the 5-HT2A/2B/2C (Porter et al., 1999) and to the 5-HT5A/5B/6/7 receptors (Matthes et al., 1993; Monsma et al., 1993; Ruat et al., 1993) but does not bind to the 5-HT3/4 receptors (Gerald et al., 1995; Peroutka & Hamik, 1988). All of the phenethylamine hallucinogens studied only bind to the 5-HT2A/2B/2C receptors (Adham et al., 1993; Erlander et al., 1993; Lovenberg et al., 1993a; Nelson et al., 1999; Pierce & Peroutka, 1989; Titeler et al., 1988; Zgombick et al., 1992). Given that these 5-HT2 receptors are the only shared targets between LSD and the phenethylamine hallucinogens and that the 5-HT2B receptor is only expressed very weakly in the brain (Pompeiano et al., 1994; Schmuck et al., 1994), it seems that the 5-HT2A/2C receptors are the only remaining candidates as the site of hallucinogenic action for this class of drugs.
There are a number of reasons why the 5-HT2 receptor has been identified as the primary site of action for hallucinogens. First, there is a preponderance of 5-HT2 to 5-HT2C receptors in the neocortex (Pompeiano et al., 1994; Wright et al., 1995). Also, when 5-HT2 receptor antagonists are used to block the stimulus cue of the phenethylamine hallucinogen DOI (Fig. 1B) and LSD in drug discrimination studies, there is a tighter correlation between the antagonists' affinity for the 5-HT2A receptor and their ability to block the interoceptive cue than there is for the 5-HT2C receptor (Fiorella et al., 1995). This means that it appears that blocking the 5-HT2A receptor more directly blocks the effects in animals associated with hallucinogenic effects than blocking the 5-HT2C receptor. Schreiber et al. (1994) reported that they were able to block drug discrimination in rats using agonists selective for 5-HT2A but not 5-HT2C.
Further evidence for 5-HT2A's central role in hallucinogenic activity comes from research with DMT. The indole hallucinogen N,N-dimethyltryptamine (DMT) does not produce tolerance to its hallucinogenic effects in humans (Strassman, 1996) and in line with this, in cultured fibroblasts expressing 5-HT2A/2C receptors, 5-HT2A receptor does not show desensitization to DMT, but the 5-HT2C receptor does (Smith et al., 1998). Finally, in human trials, the indole hallucinogen psilocybin produced "altered states of consciousness" which were blocked by ketanserin, which has an affinity for the 5-HT2A 10-30 times that of the 5-HT2C receptor (Vollenweider et al., 1998).
5-HT2A receptor localization #
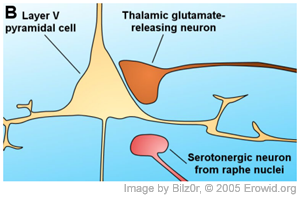
Given that the 5-HT2A receptor is the site of action for hallucinogens, an attempt to localize the receptor may give insights into how hallucinogens affect consciousness. Early studies attempting to localize the receptor involved in hallucinogen action used halogenated LSD analogues, which presumably had the same non-specific binding pattern as LSD and hence did not report 5-HT2A receptor specifically (Engel et al., 1984; McKenna & Saavedra, 1987; Wong et al., 1987). Most recent studies have used very specific antibodies to localize 5-HT2A receptors (a technique called immunocytochemistry), and generally, their results have been in complete agreement. Jakab and Goldman-Rakic (1998) examined macaque brains and reported dense 5-HT2A receptor immunoreactivity (places where the antibodies bound specifically) throughout all cortical regions, specifically in the frontal, prefrontal, temporal and occipital cortex. Throughout the cortical sheet there were two intensely stained bands, consisting of layer II and III, and layer V and VI. The cerebral cortex is a convoluted 'sheet' of brain tissue about 2-5mm thick, whose folds form the outer inch of the human brain and give the brain its signature uneven, bumpy appearance. The cerebral cortext is itself made up of layers, a feature that was described as early as 1776. The cerebral cortex (which is also referred to by its major part, the neocortex) is still usually described as being divided into six distinct layers, labeled I through VI. Although the function of the different cortical layers are not yet well identified, each layer is generally stereotyped as having inputs from certain other layers and locations in the brains, as well as outputs that reach into other layers or other areas of the brain. The possible significance of the effect on layer V pyramidal cells is commented on in the "Putting it all together" section below.
Most, if not all, of the pyramid-shaped cells (called 'pyramidal cells') in this experiment reacted to the 5-HT2A immunoassay and were 'labeled' by the antibodies as having these serotonin receptors. Many large- and medium-sized interneurons were also labeled, while most small-sized interneurons were unlabeled.
There were also a small percentage of weakly labeled presynaptic sites, often containing dense core vesicles. Miner et al. (2003) quantified the different locations of 5-HT2A receptors in the rat prefrontal cortex using various immunocytometric techniques. Out of 325 identifiable structures, it was reported that 73% of immunoreactive sites were postsynaptic, and the majority of these, extrasynaptic. Twenty-four percent of the labeled profiles were presynaptic, belonged to thin, unmyelinated axons and were often seen to contain dense core vesicles, that is to say, terminals which looked like monoaminergic neurons.
The 5-HT2A receptor is a protein, and like all other proteins is produced from messenger RNA (mRNA). By localizing mRNA, one can localize the cell bodies which produce the protein in question. This technique is called "in situ hybridization". In contrast to immunocytochemistry, in situ hydrbization doesn't tell you exactly where on the cell the protein is located, but what it tells you is which cells produced the protein in the first place. This is very useful when it comes to neurons which project long distances. For instance, neurons of the thalamus largely project to the cortex. Immunocytochemistry would just tell you that there are 5-HT2A receptors in the cortex, in situ hybridization on the other hand can tell if the receptors are on cells which originated in the thalamus. The thalamus is another area where hallucinogens are speculated to work (Lambe & Aghajanian, 2001; Marek et al., 2001) and two studies using in situ hybridization have shows that the reticular and lateral geniculate nuclei as well as the zona incerta of the thalamus all contain 5-HT2A receptor mRNA (Cyr et al., 2000; Pompeiano et al., 1994).
Modern mechanistic hallucinogen research #
A description of the research into the mechanism of hallucinogen activity in the brain is necessarily highly technical. Those unfamiliar with neurophysiology will find this section very dense and may want to skip down to the
Putting it all together conclusion where this is summarized.In slices of the cerebral cortex, the most marked result of 5-HT2A receptor activation is an increase in postsynaptic potentials, the electrical hallmarks of synaptic activity. Postsynaptic potentials essentially come in two flavours: excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs). EPSPs are "excitatory" because they bring the cell closer to firing. EPSPs are almost exclusively caused by the synaptic release of glutamate. IPSPs on the other hand are "inhibitory" because they reduce the likelihood that the cell will fire. IPSPs are caused by the synaptic release of GABA. In rat piriform cortex pyramidal cells, DOI and LSD induce IPSPs by directly exciting GABAergic interneurons (Marek & Aghajanian, 1996). However, 5-HT2A receptor activation causes different effects in other parts of the brain. In medial prefrontal cortex (mPFC) pyramidal cells, 5-HT2A receptor activation causes some IPSPs but primarily causes an increase in the amplitude and especially frequency of spontaneous EPSPs with a small effect on electrically evoked EPSPs, especially in the later phases of the EPSP (Aghajanian & Marek, 1997; Aghajanian & Marek, 1999; Marek & Aghajanian, 1999; Marek & Aghajanian, 1998).
Simply put, this research shows that activating the 2A subtype of serotonin receptor (5-HT2A) causes an increase in synaptic traffic and its role is mainly excitatory.
Applying 5-HT to cortical slices and recording activity from layer V pyramidal cells produced the most robust EPSPs in a distribution pattern closely matching the known distribution of 5-HT2A receptors. This type of test showed the highest activation in Layer I and Va (Aghajanian and Marek, 1997). If this large increase in EPSPs in the mPFC occurred in a whole animal situation (aka in vivo), one would expect to be able to record an increase in glutamate in the extracellular fluid surrounding these excited cells (as EPSPs are caused by the release of glutamate). Scrugges et al. (2003) showed this with in vivo microdialysis; showing DOI induces an increase in extracellular glutamate in the medial prefrontal cortex (mPFC) of awake animals.
Also, in two different human trials, psilocybin induced a large increase in frontal cortex metabolism with more modest increases in other cortical and subcortical areas (Gouzoulis-Mayfrank et al., 1999; Vollenweider, 2001). As firing/active neurons require more energy than inactive ones, this increase in metabolism is believed to reflect an increase in neuronal activity.
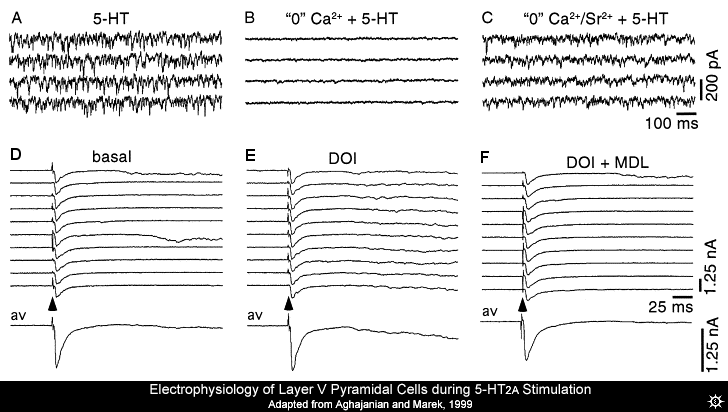
The excitatory postsynaptic potentials (EPSPs) induced by 5-HT activating the 5-HT2A receptor in mPFC pyramidal cells are Ca2+ and tetrodotoxin sensitive. Seemingly indicating that they are the product of neurons firing (as neuronal firing is blocked by tetrodotoxin) and the release of neurotransmitter-containing vesicles (a Ca2+ dependent process. Also, the EPSPs are inhibited by classical presynaptic inhibitors like the group II/III metabotropic glutamate receptor (mGluR2/3) agonists (Marek et al., 2000) and mu-opioid receptor agonists (Marek & Aghajanian, 1998). These facts together indicate that the EPSPs are not a product of postsynaptic 5-HT2A receptor activation.
On the other hand, the increase in EPSPs does not seem to be due to an increase in neighboring neurons firing and activating the cells being recorded from, as neighbouring cells have not been recorded as having action potentials when the 5-HT was directly applied (Aghajanian & Marek, 1997). As mentioned above, if the solution that the slice is perfused with to keep it alive is Ca2+ free, then all 5-HT-induced increase in EPSPs evoked by electrical stimulation (which excites neighbouring cells) and spontaneous EPSPs were blocked, but if Ca2+ was replaced with Sr2+, the 5-HT induced increase in spontaneous EPSPs returned.
Previous experiments had only briefly looked at the effect that 5-HT2A receptor activation has on electrically evoked EPSPs, so Aghajanian and Marek (1999) examined it a bit more closely. It was noted that the increase in evoked currents caused by 5-HT2A receptor activation induced by DOI were found largely after the classical electrically evoked excitatory postsynaptic potentials (direct stimulation using micro-electrodes). This is shown as the slow sag in current after the quick dip (EPSC) in current in Fig. 3E. (Aghajanian & Marek, 1998; Aghajanian & Marek, 1999). Both the spontaneous and evoked EPSPs had all the hallmarks of "asynchronous" transmitter release; a form of release that happens after the action potential has caused classical synchronous or phasic transmitter release, that can be evoked in solutions where Ca2+ is replaced with Sr2+ (Kirischuk & Grantyn, 2003; Rumpel & Behrends, 1999). Thus, it was hypothesized that hallucinogens had their effect by increasing asynchronous transmission via presynaptic 5-HT2A receptors on glutamatergic terminals.
Signal amplification #
Another effect of 5-HT2A receptor activation in layer V medial pre-frontal cortex (mPFC) cells is an enhancement of a voltage-sensitive persistent sodium current (INaP) (Aghajanian and Marek; 1997). This means that whenever excitatory currents bring a cell closer to its threshold for firing, a supplementary current will be induced, effectively amplifying the synaptically induced current. This may in part explain the increase in EPSP amplitude seen during application of 5-HT2A agonists.
The search for the source of the increased excitation caused by hallucinogens #
One way to try to locate which neurons in the brain are responsible for the activity is to destroy specific areas in the brains of rats and mice and then test them to see what changes have occured.Marek et al., (2001) lesioned (damaged/destroyed) the medial thalamus and amygdala of rats in an attempted to define the location and source of the 5-HT2A receptors which are activated by their agonists and cause increases spontaneous EPSPs in the layer V of the medial prefrontal cortex (mPFC). The neurotoxic chemical N-methyl-D-aspartate (NMDA) was infused by cannula into the brains of living animals in order to lesion the midline and intralaminar nucleus of the thalamus, and using radiofrequencies, the basolateral nucleus of the amygdala was lesioned. These nuclei were chosen because they are classical sources of excitatory input into the mPFC and they are one of the few nuclei whose projections share the laminar pattern 5-HT2A receptors distribution in the mPFC (Bacon et al., 1996; Berendse & Groenewegen, 1991).
Roughly 18 days after the damage, the animals were sacrificed and cortical slices were taken to ensure the accuracy of the lesion, electrophysiological work was done and 5-HT2A, mu-opioid and mGluR2/3 receptors were imaged via autoradiography. It was reported that the thalamic lesions were largely specific to the basolateral, midline and intralaminar nuclei, and where they weren't, the damage spilled onto thalamic nuclei with no input to layer V. In cortical slices where the midline and intralaminar nuclei were lesioned, there was no change in spontaneous or chemically evoked EPSPs recorded in mPFC pyramidal neurons, indicating that there was no change in the mPFCs sensitivity to excitatory input. However, there was 60% decrease in the frequency of 5-HT induced EPSPs. Lesions of the amygdala had no effect on 5-HT induced EPSPs. Lesions to the thalamus lead to a decrease in mGluR2/3 (~20%) and mu-opioid receptor binding (20-50%) throughout the layers of the cortex, but an increase in 5-HT2A receptor binding.
These results, although not conclusive, give weight to several ideas. For one, the dramatic decrease in 5-HT2A activation-mediated EPSPs in mPFC pyramidal cells caused by midline and intralaminar lesions indicate that these nuclei, but not the basolateral nucleus of the amygdala, are a major source of the cortical afferents responsible for the EPSPs. It had previously been shown that mGluR2/3, and mu-opioid receptor activation inhibits 5-HT2A mediated EPSPs, the decrease in their density after thalamic lesioning indicated that some of these receptors were located on the thalamocortical neurons. Finally, the fact that thalamic lesions caused a significant decrease in EPSP frequency yet caused an increase in 5-HT2A receptor density, indicates that there is a very limited amount, if any 5-HT2A receptors on the projection of midline and intralaminar neurons in the cortex. Therefore it is very unlikely that 5-HT2A receptors on the presynaptic terminals of thalamocortical neurons are responsible for the increase in EPSPs in mPFC pyramidal cells caused by 5-HT2A receptor agonists.
Apparent paradox of 5-HT presynaptic activity #
These results with the other research looking at the 5-HT hallucinogens are somewhat paradoxical: a presynaptic action of 5-HT2A receptors on presynaptic terminals devoid of 5-HT2A receptors. One way to avoid the paradox would be a retrograde messenger. Conceivably, 5-HT2A receptor activation on the postsynaptic terminal could lead to the generation of a retrograde messenger, which could activate the presynaptic terminal. This possibility gained more ground after Lambe and Aghajanian (2001) showed the similarity between the EPSPs induced by 5-HT2A receptor activation and antagonists of Kv1.2- and possibly Kv3-containing potassium channels, channels which are blocked by the retrograde messenger arachidonic acid (Poling et al., 1995; Poling et al., 1996). It was reported that there was a correlation between the affinity of various potassium channel antagonists for Kv1.2-containing channels and their ability to induce EPSPs in layer V mPFC pyramidal cells. There were many similarities between the EPSPs induced by the Kv1.2 antagonist mu-dendrotoxin (DTX) and 5-HT. The EPSP traces recorded looked similar in both amplitude and frequency; they were induced most strongly in the same layers of the cortex and they were inhibited by mu-opioid agonists and thalamic lesions. Importantly, 5-HT induced EPSPs were only slightly additive with DTX-induced EPSPs, and when DTX was added with tetraethylammonium (TEA), a general potassium channel antagonist, 5-HT could not induced any more EPSPs at all.
To exclude the possibility of a ceiling effect, it was shown that nicotine induced EPSPs were completely additive with DTX induced EPSPs. It is believed that the other potassium channel that were blocked by TEA are Kv3.2-containing channels, as it is highly expressed in thalamic neurons (Weiser et al., 1994) and its expression is highly decreased by thalamic lesions (Moreno et al., 1995). Interestingly, the Kv1.2 and Kv3.2 are the only two potassium channels which have been shown to be inhibited by arachidonic acid, a product of 5-HT2A receptor activation (discussed below) (Poling et al., 1995; Poling et al., 1996).
Blockade of presynaptic potassium channels causes depolarization that leads to an increase in intracellular Ca2+ concentration and hence increases the rate at which neurotransmitters are spontaneously released. Despite conventional thinking, this effect has been shown to be voltage sensitive sodium channel dependent (Lambe & Aghajanian, 2001; Sanchez-Prieto et al., 1996) and may explain why the increase in EPSPs caused by 5-HT2A receptor activation is TTX-sensitive (a blocker of voltage sensitive sodium channels). Arachidonic acid can be produced via the action of phospholipase A2 (PLA2), an enzyme that 5-HT2A receptor activation is known to stimulate along with its classical linkage to phospholipase C (PLC) (Kurrasch-Orbaugh et al., 2003). Therefore, it was hypothesized that the EPSPs induced by 5-HT2A receptor agonists are caused by the activation of postsynaptic 5-HT2A receptors, the production of arachidonic acid by PLA2, and its action on presynaptic potassium channels.
Potencies and second messengers #
This hypothesis raises an interesting problem; although hallucinogens' behavioral potencies are tightly correlated with their 5-HT2A receptor affinity, there seems to be no correlation between behavioral potencies and their ability to stimulate second messenger production (Nichols, 2004). For example, LSD is approximately 20 times more potent than the hallucinogenic amphetamine DOB in drug discrimination experiments (Glennon et al., 1984a). But in mouse fibroblasts, DOB is slightly more potent than LSD at inducing arachidonic acid release, and only around seven times less potent than LSD at stimulating PLC-mediated inositol phosphate production. It is also possible that one second messenger is 'pro-hallucinogenic' while another is 'anti-hallucinogenic'. This theory could explain the lack of correlation between second messenger production and behavioral effect, as it would not be the potency of a drug at stimulating second messenger production, but the ratio of the potency at producing one second messenger to another. On top of that, the 5-HT2A receptor pathway has one more card up its sleeve, as it has been shown to be strongly linked to phospholipase D (PLD) in a ADP-ribosylation factor-dependent manner (Mitchell et al., 1998; Robertson et al., 2003). Unfortunately, no studies have looked at the ability of hallucinogens to activate PLD.The situation becomes more difficult still when one tries to include the results of Puig et al., (2003). In that research, DOI (50-300åµg/kg I.V.) was given to anaesthetized animals and the firing rates of mPFC neurons were recorded. It was shown, as expected from the above results, that DOI increased the overall firing rates 238% in a largely 5-HT2A receptor dependent manner (DOI excited 38% of neurons, on average 481%, 32% were unaffected and 30% were inhibited, on average 11%). However the DOI-induced increase in firing rate was not inhibited by mu-opioid or mGluR2/3 agonists or lesions to the midline and intralaminar thalamic nuclei (i.e. the same nuclei which Marek et al. (2001) lesioned and reported that 5-HT2A-mediated spontaneous EPSPs were massively reduced).
Interestingly, injection of excitatory drugs (the GABA-A antagonist bicuculline) into the thalamus produced an increase in cellular firing which physiologically and pharmacologically mirrored the EPSPs of Aghajanian and Marek: they were reduced by mu-opioid or mGlurR2/3 agonists and would presumably be blocked by thalamic lesions. Exactly what these findings say about those put forward by Aghajanian and Marek is unclear. They could suggest that the excitatory drive is actually derived from cortico-cortical projections, something which is supported by Aghajanian and Marek's (1998; 1999) work on electrically evoked EPSPs (the evoked EPSPs were caused by stimulating the majority of inputs onto the cell, most of which were excitatory cortico-cortical projections).
Could hallucinogens act by decreasing excitation? #
While there is an overarching theme that hallucinogens act by increasing excitatory cortical transmission, two papers challenge this classical stance with evidence that
5-HT2A receptor agonists inhibit N-methyl-D-aspartate (NMDA)
receptor-mediated currents (Arvanov et al., 1999a; Arvanov et al., 1999b). It was
reported that in layer V pyramidal cells in mPFC slices, DOB at low concentrations
(0.01-1åµM) caused an increase in the currents induced by perfusion with 10µM of NMDA. On the other hand at high concentrations (>1µM) it produced a Ca2+/calmodulin kinase
II-dependent blockade of NMDA-induced currents. The potentiating effect of DOB was Ca2+ sensitive, and presumed to be of presynaptic origin, in line with the above research. The AMPA receptor antagonist CNQX was added, to block the effect of any spontaneously released glutamate, and it was then seen that even low concentrations of DOB would decrease NMDA-induced currents, with an IC50 of 130nM (IC50 is the "inhibitory concentration 50%", or the concentration necessary to cause 50% inhibition). This was repeated with LSD, and it was shown to have an IC50 of 9nM. The authors stated that the concentration of LSD needed to induce an enhancing effect on NMDA induced currents was >300nM and far above the 10-20nM plasma concentration found in recreational use (Aghajanian & Bing, 1964; Hawks & Chiang, 1986), and hence unphysiological.
However, it should be noted that although potentiation of NMDA receptor-mediated currents seems unlikely to be a cause of the hallucinogenic action of 5-HT2A receptor agonists, that does not say they can not act by causing glutamate release as described above. Indeed, if hallucinogens worked solely by inhibiting the NMDA receptor in some fashion, one would expect hallucinogens to generalize to NMDA-receptor antagonists in drug discrimination experiments, but this has not been shown consistently (Jones et al., 1998; West et al., 2000). Furthermore, if inhibiting the NMDA glutamate receptor was the complete explanation of hallucinogen action, it seems surprising that mGluR2/3 agonists, which inhibit 5-HT2A receptor-mediated glutamate release in vitro, also inhibit behavioral aspects of hallucinogens such as head shakes (Klodzinska et al., 2002) and drug-induced stimulus control (Winter et al., 2004). However it seems possible that hallucinogens work by both increasing glutamate release and inhibiting NMDA-receptors. Indeed, if hallucinogens have varying abilities to inhibit NMDA-receptors and induce EPSPs in cortical pyramidal cells, it could help explain how different hallucinogens seem to produce the wide ranging subjective effects reported by recreational users (Shulgin & Shulgin, 1991; Shulgin & Shulgin, 1997).
Locus coeruleus activity #
Another possibly important effect of hallucinogens is their ability to alter the firing of the locus coeruleus (LC), the major source of noradrenaline (NA) in the brain.
Systemic administration of either phenethylamine or indole hallucinogens to anaesthetized animals causes a 5-HT2A receptor dependent decrease in the
spontaneous activity of the LC, but an increase in activity evoked by sensory stimulation (Aghajanian, 1980; Rasmussen & Aghajanian, 1986; Rasmussen et al., 1986).
This effect was shown to be mediated by 5-HT2A receptors extrinsic to the LC, as hallucinogens microiontophoretically applied to LC cell bodies did not produce this
effect. The ability of 5-HT2A receptor agonists to inhibit LC firing was blocked by local administration of GABAA antagonists and the stimulatory action of
hallucinogens on LC activity was blocked by NMDA receptor antagonists, but not non-NMDA ionotropic glutamate receptor antagonists (Chiang & Aston-Jones, 1993). The LC has been likened to a "novelty detector" due to its increased activity in response to novel stimuli. The release of NA also seems to gate sensory information to a degree, and to increase the signal to noise ratio, by inhibiting basal neuronal activity but increasing responses to sensory stimulation (reviewed by Sara et al., 1994; Woodward et al., 1991). Interestingly, the laminar distribution of the alpha1-adrenoreceptor closely matches that of the 5-HT2A receptor (Palacios et al., 1987), and NA induces a alpha1-adrenoreceptor-dependent increase in EPSPs in layer V mPFC cells in a similar fashion to 5-HT (Marek & Aghajanian, 1999). It seems possible then that the increase in sensory-evoked LC firing could produce some of the cognitive effects induced by hallucinogens, such as ordinary objects appearing fascinating.
Putting it all together #
Unfortunately, the majority of recent research into hallucinogens has focused on isolated brain regions, specifically using in vitro cortical brain slices. This
preparation is usually devoid of the spontaneous activity seen in the neocortex of an intact animal. Although one can replicate the slow spontaneous rhythmic activity seen in
vivo if the bathing medium closely matches the extracellular ionic composition seen in the brain in situ, one can not replace the cortical and subcortical inputs lost
when the section is taken from the brain (Sanchez-Vives & McCormick, 2000). Indeed, these subcortical and especially the thalamic inputs seem critical to this complex
activity when it is hypothesized that thalamic projections are the neurons that supply the increased excitatory input in response to hallucinogens. Furthermore, cortical input
from the locus coeruleus and the raphe nuclei are lost. Yet one can attempt to build a larger picture out of the available research.As reviewed above, the medial prefrontal cortex (mPFC) cells that seem to be excited most by application of 5-HT2A receptor agonists are layer V pyramidal cells. In a theory reviewed by Jones (2002), layer V pyramidal cells have been implicated in the binding of separate sensory stimuli into a discrete conscious event. Major sensory stimuli often lead to high frequency, 20-50Hz "gamma" oscillatory firing between thalamic relay cells and the area of cortex that they project to. These gamma oscillations are dependent on thalamo-cortico-thalamic circuits, and some are believed to code the conscious recognition of the stimuli (Golshani & Jones, 1999; Steriade & Amzica, 1996). This loop is created by thalamic relay "core" cells in specific thalamic nuclei activating layer VI pyramidal cells, and their projections back to the core cells. This loop system would stay in the cortical columns originally activated by the thalamic relay cell, if it did not also activate layer V pyramidal cells. Layer V cells have diverse intracortical projections and, importantly, project to many non-specific thalamic nuclei. Therefore these would "decide" which distant cortical areas could be activated by the original stimulus.
This way, associated stimuli could be bound to form synchronous activity across the cortex. By activating layer V pyramidal cells' coritco-cortical and cortical-thalamic projections, hallucinogens could cause the spread of high-frequency oscillations to areas that would not normally be activated. In sensory cortexes this could produce effects such as the hallucinations and synesthesia. In the frontal cortex the spreading of high frequency oscillations could result in mood changes and alteration to ego perception. Indeed this theory can be used to explain any facet of the hallucinogenic experience where a stimulus produces an inappropriate perception (e.g. an object being recognized as something it is not, a sound being heard as something it is not, though not simple visual effects like fractals). It is interesting to note that the frequency of excitatory postsynaptic potentials (EPSPs) induced by 5-HT2A receptor agonists was shown to be 36Hz (Aghajanian & Marek, 1998), right in the middle of the gamma frequency, (explained above).
Other 'big picture' theories of hallucinogen action involve their potential action on the thalamic reticular nucleus (TRN). The TRN is thought to work as a filter for transmission of information from the thalamus to the cortex (reviewed by Guillery & Harting, 2003). TRN neurons are nearly exclusively GABAergic, and can switch thalamic relay cells from tonic firing mode to burst firing mode. In tonic mode, the relay cells can accurately transmit sensory information because their firing rate closely reflects the rate and amplitude of excitatory inputs while cells in burst firing mode reach a maximum firing rate when inputs reach 15Hz at rates above 100Hz, but cells in burst fire mode can not fire faster than 15Hz and hence the integrity of information is lost (Kim & McCormick, 1998; McCormick & Feeser, 1990).
It is generally believed that bursts act as some kind of "wake-up call" to the cortex, as bursts are usually firing in response to a new stimulus (Grubb & Thompson, 2005). However, this could just be because bursts are initiated when a cell is excited out of an unexcited (hyperpolarized) state, the kind of state a neuron would be in between presentations of stimuli in an experimental setting i.e. in an anaesthetized animal. Because there is a large difference in the neurophysiological consequences caused by different patterns of cell firing and certain firing modes predominate during different behavioural states (tonic firing is more common in awake animals while burst firing is more common during sleep) (Steriade & Llinas, 1988), it seems unlikely that there is no functional difference between these two firing modes.
In a model proposed by Vollenweider and Geyer (2001) activation of 5-HT2A receptors on TRN neurons could activate them in the same way that 5-HT2A receptors activate GABAergic neurons in the cortex, and decrease the ability of the TRN to gate information flow effectively.
This author notes that the zona incerta of the thalamus also expressed 5-HT2A receptors (Pompeiano et al., 1994), and projects GABAergic neurons to higher-order thalamic relay cells (Bartho et al., 2002). The zona incerta, unlike most thalamic nuclei, receives its cortical input from almost exclusively layer V pyramidal cells (Bartho et al., 2002; Mitrofanis & Mikuletic, 1999), and this is another way by which hallucinogens could effect cortical function.
Along with any cortico-thalamo-cortical action of hallucinogens, their action in potentiating locus coeruleus (LC) firing in response to sensory stimuli will also excite cortical cells, possibly in a synergistic fashion to 5-HT2A receptor stimulation. The idea that hallucinogens work at least in part by inhibiting firing of cells in the raphe nuclei was once seen as a major hypothesis, but now seems unlikely, thought it may modulate the subjective effect hallucinogens produce. Unfortunately, in the end, most of these theories are speculative rather than being scientific hypthoses based on empirical observations, as there is only a very limited amount of research on hallucinogens and most of that research is restricted to in vitro electrophysiology on cortical cells. Future research needs to look into the effect that hallucinogens have on thalamic cells and thalamocortical oscillations. Since the intracellular cascade responsible for hallucinogen action is not yet fully understood, it is hard to imagine science being able to explain the much more complicated details of hallucinogens' action on consciousness.
References #
- # ADHAM N, KAO HT, SCHECTER LE, BARD J, OLSEN M, URQUHART D, DURKIN M, HARTIG PR, WEINSHANK RL, BRANCHEK TA. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci U S A. 1993 ; 90 408-12. [ Search ]
- # AGHAJANIAN GK. Mescaline and LSD facilitate the activation of locus coeruleus neurons by peripheral stimuli. Brain Res. 1980 ; 186 492-8. [ Search ]
- # AGHAJANIAN GK, BING OH. Persistence of Lysergic Acid Diethylamide in the Plasma of Human Subjects. Clin Pharmacol Ther. 1964 ; 10 611-4. [ Abstract ]
- # AGHAJANIAN GK, FOOTE WE, SHEARD MH. Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Exp Ther. 1970 ; 171 178-87. [ Abstract ]
- # AGHAJANIAN GK, FOOTE WE, SHEARD MH. Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science. 1968 ; 161 706-8. [ Abstract ]
- # AGHAJANIAN GK, HAIGLER HJ, BLOOM FE. Lysergic acid diethylamide and serotonin: direct actions on serotonin-containing neurons in rat brain. Life Sci I. 1972 ; 11 615-22. [ Abstract ]
- # AGHAJANIAN GK, HAILGLER HJ. Hallucinogenic indoleamines: Preferential action upon presynaptic serotonin receptors. Psychopharmacol Commun. 1975 ; 1 619-29. [ Search ]
- # AGHAJANIAN GK, MAREK GJ. Serotonin 5-HT2A receptors enhance asynchronous excitatory transmission in pyramidal cells (layer V) of prefrontal cortex. Soc Neurosci Abs. 1998 ; 24 1366. [ Search ]
- # AGHAJANIAN GK, MAREK GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997 ; 36 589-99. [ Search ]
- # AGHAJANIAN GK, MAREK GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999 ; 825 161-71. [ Search ]
- # ANDEN NE, CORRODI H, FUXE K. Evidence for a central 5-hydroxytryptamine receptor stimulation by lysergic acid diethylamide or bufotenin. Br J Pharmacol. 1968 ; 34 1-7. [ Abstract ]
- # ARVANOV VL, LIANG X, MAGRO P, ROBERTS R, WANG RY. A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci. 1999a ; 11 2917-34. [ Search ]
- # ARVANOV VL, LIANG X, RUSSO A, WANG RY. LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex. Eur J Neurosci. 1999b ; 11 3064-72. [ Search ]
- # BACON SJ, HEADLAM AJ, GABBOTT PL, SMITH AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 1996 ; 720 211-9. [ Search ]
- # BARTHO P, FREUND TF, ACSADY L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002 ; 16 999-1014. [ Search ]
- # BERENDSE HW, GROENEWEGEN HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991 ; 42 73-102. [ Search ]
- # CERKETTI A, ROTHLIN E. Role of 5-hydroxytryptamine in mental disease and its antagonism to lysergic acid derivatives. Nature. 1955 ; 176 785-786. [ Search ]
- # CHIANG C, ASTON-JONES G. A 5-hydroxytryptamine2 agonist augments gamma-aminobutyric acid and excitatory amino acid inputs to noradrenergic locus coeruleus neurons. Neuroscience. 1993 ; 54 409-20. [ Search ]
- # Clark LC Jr, Fox RP, Morin R, Benington F. Effects of psychotomimetic compounds on certain oxidative and hydrolytic enzymes in mammalian brain. J Nerv Ment Dis. 1956 Nov;124(5):466-72. [ href="/references/refs.php?A=SearchOrGoPubMed&FirstAuthor=Clark&Y1=1956&Title=psychotomimetic">Search ]
- # CYR M, LANDRY M, DI PAOLO T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology. 2000 ; 23 69-78. [ Search ]
- # DE MONTIGNY C, AGHAJANIAN GK. Preferential action of 5-methoxytryptamine and 5-methoxydimethyltryptamine on presynaptic serotonin receptors: A comparative iontophoretic study with LSD and serotonin. Neuropharmacology. 1977 ; 16 811-818. [ Search ]
- # ENGEL G, MULLER-SCHWEINITZER E, PALACIOS JM. 2-[125Iodo]LSD, a new ligand for the characterisation and localisation of 5-HT2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1984 ; 325 328-36. [ Abstract ]
- # ERLANDER MG, LOVENBERG TW, BARON BM, DE LECEA L, DANIELSON PE, RACKE M, SLONE AL, SIEGEL BW, FOYE PE, CANNON K, ET AL. Two members of a distinct subfamily of 5-hydroxytryptamine receptors differentially expressed in rat brain. Proc Natl Acad Sci U S A. 1993 ; 90 3452-6. [ Search ]
- # FIORELLA D, RABIN RA, WINTER JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology (Berl). 1995 ; 121 347-56. [ Search ]
- # GADDUM JH. Antagonism between lysergic acid diethylamide and 5-hydroxytryptamine. J Physiol. 1953 ; 121 15. [ Abstract ]
- # GADDUM JH, HAMEED KA. Drugs which antagonize 5-hydroxytryptamine. Br J Pharmacol. 1954 ; 9 240-248. [ Abstract ]
- # GERALD C, ADHAM N, KAO HT, OLSEN MA, LAZ TM, SCHECHTER LE, BARD JA, VAYSSE PJ, HARTIG PR, BRANCHEK TA, ET AL. The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. Embo J. 1995 ; 14 2806-15. [ Search ]
- # GLENNON RA, TITELER M, MCKENNEY JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984a ; 35 2505-11. [ Search ]
- # GLENNON RA, TITELER M, YOUNG R. Structure-activity relationships and mechanism of action of hallucinogenic agents based on drug discrimination and radioligand binding studies. Psychopharmacol Bull. 1986 ; 22 953-8. [ Search ]
- # GLENNON RA, YOUNG R, HAUCK AE, MCKENNEY JD. Structure-activity studies on amphetamine analogs using drug discrimination methodology. Pharmacol Biochem Behav. 1984b ; 21 895-901. [ Search ]
- # GLENNON RA, YOUNG R, ROSECRANS JA. Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol. 1983 ; 91 189-96. [ Search ]
- # GOGERTY JH, DILLE JM. Pharmacology of D-lysergic acid morpholide (LSM). J Pharmacol Exp Ther. 1957 ; 120 340-348. [ Abstract ]
- # GOLSHANI P, JONES EG. Synchronized paroxysmal activity in the developing thalamocortical network mediated by corticothalamic projections and "silent" synapses. J Neurosci. 1999 ; 19 2865-75. [ Search ]
- # GOUZOULIS-MAYFRANK E, SCHRECKENBERGER M, SABRI O, ARNING C, THELEN B, SPITZER M, KOVAR KA, HERMLE L, BULL U, SASS H. Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind. 1999 ; placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology 20:565-81. [ Abstract ]
- # GUILLERY RW, HARTING JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol. 2003 ; 463 360-71. [ Search ]
- # HAWKS RL, CHIANG CN. Urine Testing for Drugs of Abuse. 1986 ; HAWKS, R.L. & CHIANG, C.N 1986. [ Search ]
- # HOFFER A. A program for the treatment of alcoholism: LSD, malvaria and nicotinic acid. 1967 ; HOFFER, A 1967. [ Abstract ]
- # HOYER D. Functional correlates of serotonin 5-HT1 recognition sites. J Recept Res. 1988 ; 8 59-81. [ Search ]
- # JAKAB RL, GOLDMAN-RAKIC PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998 ; 95 735-40. [ Search ]
- # JONES EG. Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci. 2002 ; 357 1659-73. [ Search ]
- # JONES HE, LI H, BALSTER RL. Failure of ibogaine to produce phencyclidine-like discriminative stimulus effects in rats and monkeys. Pharmacol Biochem Behav. 1998 ; 59 413-8. [ Search ]
- # KIM U, MCCORMICK DA. The functional influence of burst and tonic firing mode on synaptic interactions in the thalamus. J Neurosci. 1998 ; 18 9500-16. [ Search ]
- # KIRISCHUK S, GRANTYN R. Intraterminal Ca2+ concentration and asynchronous transmitter release at single GABAergic boutons in rat collicular cultures. J Physiol. 2003 ; 548 753-64. [ Search ]
- # KLODZINSKA A, BIJAK M, TOKARSKI K, PILC A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav. 2002 ; 73 327-32. [ Search ]
- # KURRASCH-ORBAUGH DM, PARRISH JC, WATTS VJ, NICHOLS DE. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem. 2003 ; 86 980-91. [ Search ]
- # LAMBE EK, AGHAJANIAN GK. The role of Kv1. 2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci. 2001 ; 21 9955-63. [ Search ]
- # LOVENBERG TW, BARON BM, DE LECEA L, MILLER JD, PROSSER RA, REA MA, FOYE PE, RACKE M, SLONE AL, SIEGEL BW, ET AL. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993a ; 11 449-58. [ Search ]
- # LOVENBERG TW, ERLANDER MG, BARON BM, RACKE M, SLONE AL, SIEGEL BW, CRAFT CM, BURNS JE, DANIELSON PE, SUTCLIFFE JG. Molecular cloning and functional expression of 5-HT1E-like rat and human 5-hydroxytryptamine receptor genes. Proc Natl Acad Sci U S A. 1993b ; 90 2184-8. [ Search ]
- # MAREK GJ, AGHAJANIAN GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999 ; 367 197-206. [ Search ]
- # MAREK GJ, AGHAJANIAN GK. 5-Hydroxytryptamine-induced excitatory postsynaptic currents in neocortical layer V pyramidal cells: suppression by mu-opiate receptor activation. Neuroscience. 1998 ; 86 485-97. [ Search ]
- # MAREK GJ, AGHAJANIAN GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther. 1996 ; 278 1373-82. [ Search ]
- # MAREK GJ, WRIGHT RA, GEWIRTZ JC, SCHOEPP DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001 ; 105 379-92. [ Search ]
- # MAREK GJ, WRIGHT RA, SCHOEPP DD, MONN JA, AGHAJANIAN GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000 ; 292 76-87. [ Search ]
- # MARTIN-RUIZ R, PUIG MV, CELADA P, SHAPIRO DA, ROTH BL, MENGOD G, ARTIGAS F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001 ; 21 9856-66. [ Search ]
- # MATTHES H, BOSCHERT U, AMLAIKY N, GRAILHE R, PLASSAT JL, MUSCATELLI F, MATTEI MG, HEN R. Mouse 5-hydroxytryptamine5A and 5-hydroxytryptamine5B receptors define a new family of serotonin receptors: cloning, functional expression, and chromosomal localization. Mol Pharmacol. 1993 ; 43 313-9. [ Search ]
- # MCCORMICK DA, FEESER HR. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience. 1990 ; 39 103-13. [ Search ]
- # MCKENNA DJ, SAAVEDRA JM. Autoradiography of LSD and 2,5-dimethoxyphenylisopropylamine psychotomimetics demonstrates regional, specific cross-displacement in the rat brain. Eur J Pharmacol. 1987 ; 142 313-5. [ Search ]
- # MINER LA, BACKSTROM JR, SANDERS-BUSH E, SESACK SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003 ; 116 107-17. [ Search ]
- # MITCHELL R, MCCULLOCH D, LUTZ E, JOHNSON M, MACKENZIE C, FENNELL M, FINK G, ZHOU W, SEALFON SC. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998 ; 392 411-4. [ Search ]
- # MITROFANIS J, MIKULETIC L. Organisation of the cortical projection to the zona incerta of the thalamus. J Comp Neurol. 1999 ; 412 173-85. [ Search ]
- # MONSMA FJ, JR, SHEN Y, WARD RP, HAMBLIN MW, SIBLEY DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol. 1993 ; 43 320-7. [ Search ]
- # MORENO H, KENTROS C, BUENO E, WEISER M, HERNANDEZ A, VEGA-SAENZ DE MIERA E, PONCE A, THORNHILL W, RUDY B. Thalamocortical projections have a K+ channel that is phosphorylated and modulated by cAMP-dependent protein kinase. J Neurosci. 1995 ; 15 5486-501. [ Search ]
- # NELSON DL, LUCAITES VL, WAINSCOTT DB, GLENNON RA. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999 ; 359 1-6. [ Search ]
- # NICHOLS DE. Hallucinogens. Pharmacol Ther. 2004 ; 101 131-81. [ Abstract ]
- # PALACIOS JM, HOYER D, CORTES R. alpha 1-Adrenoceptors in the mammalian brain: similar pharmacology but different distribution in rodents and primates. Brain Res. 1987 ; 419 65-75. [ Search ]
- # PEROUTKA SJ, HAMIK A. [3H]quipazine labels 5-HT3 recognition sites in rat cortical membranes. Eur J Pharmacol. 1988 ; 148 297-9. [ Search ]
- # PIERCE PA, PEROUTKA SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology (Berl). 1989 ; 97 118-22. [ Search ]
- # POLING JS, KARANIAN JW, SALEM N, JR, VICINI S. Time- and voltage-dependent block of delayed rectifier potassium channels by docosahexaenoic acid. Mol Pharmacol. 1995 ; 47 381-90. [ Search ]
- # POLING JS, ROGAWSKI MA, SALEM N, JR, VICINI S. Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology. 1996 ; 35 983-91. [ Search ]
- # POMPEIANO M, PALACIOS JM, MENGOD G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994 ; 23 163-78. [ Search ]
- # PORTER RH, BENWELL KR, LAMB H, MALCOLM CS, ALLEN NH, REVELL DF, ADAMS DR, SHEARDOWN MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999 ; 128 13-20. [ Search ]
- # PUIG MV, CELADA P, DIAZ-MATAIX L, ARTIGAS F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003 ; 13 870-82. [ Search ]
- # RASMUSSEN K, AGHAJANIAN GK. Effect of hallucinogens on spontaneous and sensory-evoked locus coeruleus unit activity in the rat: reversal by selective 5-HT2 antagonists. Brain Res. 1986 ; 385 395-400. [ Search ]
- # RASMUSSEN K, GLENNON RA, AGHAJANIAN GK. Phenethylamine hallucinogens in the locus coeruleus: potency of action correlates with rank order of 5-HT2 binding affinity. Eur J Pharmacol. 1986 ; 132 79-82. [ Search ]
- # ROBERTSON DN, JOHNSON MS, MOGGACH LO, HOLLAND PJ, LUTZ EM, MITCHELL R. Selective interaction of ARF1 with the carboxy-terminal tail domain of the 5-HT2A receptor. Mol Pharmacol. 2003 ; 64 1239-50. [ Search ]
- # ROGAWSKI MA, AGHAJANIAN GK. Response of central monoaminergic neurons to lisuride: comparison with LSD. Life Sci. 1979 ; 24 1289-97. [ Abstract ]
- # RUAT M, TRAIFFORT E, LEURS R, TARDIVEL-LACOMBE J, DIAZ J, ARRANG JM, SCHWARTZ JC. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci U S A. 1993 ; 90 8547-51. [ Search ]
- # RUCK CA, BIGWOOD J, STAPLES D, OTT J, WASSON RG. Entheogens. J Psychedelic Drugs. 1979 ; 11 145-146. [ Search ]
- # RUMPEL E, BEHRENDS JC. Sr2+-dependent asynchronous evoked transmission at rat striatal inhibitory synapses in vitro. J Physiol. 1999 ; 514 ( Pt 2) 447-58. [ Search ]
- # SANCHEZ-VIVES MV, MCCORMICK DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000 ; 3 1027-34. [ Search ]
- # SANDERS-BUSH E, BURRIS KD, KNOTH K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988 ; 246 924-8. [ Search ]
- # SARA SJ, VANKOV A, HERVE A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994 ; 35 457-65. [ Search ]
- # SCHMUCK K, ULLMER C, ENGELS P, LUBBERT H. Cloning and functional characterization of the human 5-HT2B serotonin receptor. FEBS Lett. 1994 ; 342 85-90. [ Search ]
- # SCHREIBER R, BROCCO M, MILLAN MJ. Blockade of the discriminative stimulus effects of DOI by MDL 100,907 and the 'atypical' antipsychotics, clozapine and risperidone. Eur J Pharmacol. 1994 ; 264 99-102. [ Search ]
- # SCRUGGS JL, SCHMIDT D, DEUTCH AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett. 2003 ; 346 137-40. [ Search ]
- # SHULGIN AT, SHULGIN A. PiHKAL: A Chemical Love Story. 1991 ; SHULGIN, A.T. & SHULGIN, A 1991. [ Search ]
- # SHULGIN AT, SHULGIN A. TiHKAL: The Continuation. 1997 ; SHULGIN, A.T. & SHULGIN, A 1997. [ Search ]
- # SMITH RL, CANTON H, BARRETT RJ, SANDERS-BUSH E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav. 1998 ; 61 323-30. [ Search ]
- # STERIADE M, AMZICA F. Intracortical and corticothalamic coherency of fast spontaneous oscillations. Proc Natl Acad Sci U S A. 1996 ; 93 2533-8. [ Search ]
- # STRASSMAN RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996 ; 73 121-4. [ Abstract ]
- # TITELER M, LYON RA, GLENNON RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl). 1988 ; 94 213-6. [ Search ]
- # TRULSON ME, HEYM J, JACOBS BL. Dissociations between the effects of hallucinogenic drugs on behavior and raphe unit activity in freely moving cats. Brain Res. 1981 ; 215 275-93. [ Search ]
- # TWAROG, B.M. & PAGE, I.H. Am J Physiol. 1953 ; 175 157. [ Search ]
- # VILLALOBOS CA, BULL P, SAEZ P, CASSELS BK, HUIDOBRO-TORO JP. 4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT2A receptor antagonists in Xenopus laevis oocytes. Br J Pharmacol. 2004 ; 141 1167-74. [ Search ]
- # VOLLENWEIDER FX. Brain mechanisms of hallucinogens and entactogens. Dialogues Clin Neurosci. 2001 ; 3 265-279. [ Search ]
- # VOLLENWEIDER FX, GEYER MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001 ; 56 495-507. [ Search ]
- # VOLLENWEIDER FX, VOLLENWEIDER-SCHERPENHUYZEN MF, BABLER A, VOGEL H, HELL D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998 ; 9 3897-902. [ Search ]
- # VOTAVA Z, PODVALOVA I, SEMONSKY M. Studies on the pharmacology of D-lysergic acid cycloalklamdes. Arch Int Pharmacodyn Ther. 1958 ; 115 114-130. [ Abstract ]
- # WEISER M, VEGA-SAENZ DE MIERA E, KENTROS C, MORENO H, FRANZEN L, HILLMAN D, BAKER H, RUDY B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994 ; 14 949-72. [ Search ]
- # WEST WB, LOU A, PECHERSKY K, CHACHICH ME, APPEL JB. Antagonism of a PCP drug discrimination by hallucinogens and related drugs. Neuropsychopharmacology. 2000 ; 22 618-25. [ Search ]
- # WINTER JC, ECKLER JR, RABIN RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl). 2004 ; 172 233-40. [ Search ]
- # WONG DF, LEVER JR, HARTIG PR, DANNALS RF, VILLEMAGNE V, HOFFMAN BJ, WILSON AA, RAVERT HT, LINKS JM, SCHEFFEL U, ET AL. Localization of serotonin 5-HT2 receptors in living human brain by positron emission tomography using N1-([11C]-methyl)-2-Br-LSD. Synapse. 1987 ; 1 393-8. [ Search ]
- # WOODWARD DJ, MOISES HC, WATERHOUSE BD, YEH HH, CHEUN JE. The cerebellar norepinephrine system: inhibition, modulation, and gating. Prog Brain Res. 1991 ; 88 331-41. [ Search ]
- # WOOLLEY DW, SHAW E. A biochemical and pharmacological suggestion about certain mental disorders. Science. 1954 ; 199 587-588. [ Abstract ]
- # WRIGHT DE, SEROOGY KB, LUNDGREN KH, DAVIS BM, JENNES L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995 ; 351 357-73. [ Search ]
- # ZGOMBICK JM, SCHECHTER LE, MACCHI M, HARTIG PR, BRANCHEK TA, WEINSHANK RL. Human gene S31 encodes the pharmacologically defined serotonin 5-hydroxytryptamine1E receptor. Mol Pharmacol. 1992 ; 42 180-5. [ Search ]
Notes #
- 1 - We believe that the term 'psychotomimetic' had been used before Clark in 1956, but have been unable to track down the original reference.
- 2 - In the figure shown, the X axis is log-transformed (a way of compressing large ranges into a small chart) data for tests which have shown rats will report that one drug is similar to another. The Ki values along the Y axis are log-transformed measurements of how well the compounds bind to the 5-HT2 receptor. This is to show how there appears to be a correlation between the binding of the various hallucinogens to the 5-HT2 receptors appears to correlate well with the dose at which they are active in the rats. See Rat Discrimination, ED50, Ki.
Definitions #
A term used to describe nerves and neurons that carry or transmit signals towards the brain or inside the brain away from sensory apparatus. From latin meaning 'to bring towards'.
Effective Dose 50% or the dose at which 50% of the tested population reached a certain defined level of effects with a given substance. This is generally used as a way state the 'normal' dosage level for a medicine in laboratory procedures. Differs from what is commonly know as a "threshold dose".
Sites on cells which react to and bind with certain anti-body proteins.
Neurons that are connected only to other neurons and not to sensory cells or muscles. Generally, interneurons are involved with internal signal processing.
Ki, an "inhibitory constant" is a unit of measurement for receptor binding affinity of a chemical/substance. The lower the Ki, the higher its potency. Ki is a relative value assigned to the compound when compared to a standardized ligand for the receptor, and not an absolute value. Sometimes the Ki and Km are referrred to as 'binding ratios'. See also Km.
Km, is a unit of measure related to Ki that indicates how well a chemical binds to a receptor. The higher the Km, the higher the chemical's potency.
Overly technical description follows: The basic idea is that Kd is the true measure of an affinity a ligand (chemical) has for a receptor. Kd, by definition, = Kon / Koff; where Kon = association constant of the ligand for the receptor, and Koff = dissociation constant of the ligand for the receptor. The rate of Association = Kon x the concentration of the receptor x the concentration of the ligand, while the rate of dissociation = Koff x the concentration of receptors with ligands bound to them. The unit of Kd is mol/L and in a perfect world is equal to the concentration of ligand needed to get 50% of the receptors occupied at any one time. Ki is an esstimation of Kd produced by a kind of experiment called an "inhibition binding" experiment. In the real world, there are a variety of (imperfect) ways of measuring actual binding affinity for a given chemical and receptor pair and all lead to slightly different results.
Overly technical description follows: The basic idea is that Kd is the true measure of an affinity a ligand (chemical) has for a receptor. Kd, by definition, = Kon / Koff; where Kon = association constant of the ligand for the receptor, and Koff = dissociation constant of the ligand for the receptor. The rate of Association = Kon x the concentration of the receptor x the concentration of the ligand, while the rate of dissociation = Koff x the concentration of receptors with ligands bound to them. The unit of Kd is mol/L and in a perfect world is equal to the concentration of ligand needed to get 50% of the receptors occupied at any one time. Ki is an esstimation of Kd produced by a kind of experiment called an "inhibition binding" experiment. In the real world, there are a variety of (imperfect) ways of measuring actual binding affinity for a given chemical and receptor pair and all lead to slightly different results.
Laminar simply refers to things in layers. The Laminar Pattern or Laminar Distribution of certain nerve cells indicates that they are in sheets or layers in the brain.
A relatively non-invasive technique for applying drugs and chemicals to tissue. An extremely fine glass needle filled with a drug-containing solution is positioned close to the cells in question, and a small current is passed through the solution. If the drug in question is charged it will flow with the current, out of the needle and into the tissue. Microiontophoresis can be used to eject extremely tiny amounts of solution.
A collective term for neurons that produce or are activated by the neurotransmitters serotonin, dopamine, noradrenaline and adrenaline. The term comes from the fact that these neurotransmitters share a chemical property of having a single amine.
Pyramid-shaped cells that are a common and key type of neuron in the brain.
Rats are trained to discriminate a psychoactive drug from saline (salt water). They are trained to pres one lever when they are given, for example, 2C-B and to press a different bar when they are given saline. After the rats are reliably trained to do this, the 2C-B is then replaced with the substance in question. If the rats reliably press the 2C-B lever when they are given 2C-T-7, the new substance is determined to "act similarly to 2C-B" or to "substitute" for 2C-B.
- An agonist is a substance/chemical that binds to a receptor and triggers a response in the cell.
- An antagonist is a chemical that blocks or inhibits cell mechanisms, including transporters and receptors. Antagonists can stop other chemicals from binding or being released and therefore reduce the cells' response to other chemicals or signals.
Chemicals which are created or released by the activation of receptors. These secondary-messenger chemicals mediate and activate many cellular actions. The term indicates that the 'message' between two cells or between two parts of the same cell are carried by chemicals involved through at least one other mechanism between the initiator and the triggered action.
Tetrodotoxin-sensitive