
#175 TRIS
TRESCALINE; TRISESCALINE; 3,4,5-TRIETHOXYPHENETHYLAMINE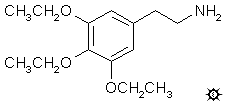
|
| [3D .mol structure] |
This product 3,4,5-triethoxybenzyl alcohol was suspended in 30 mL con-centrated HCl, heated briefly on the steam bath, cooled to room temperature, and suspended in a mixture of 75 mL CH2Cl2 and 75 mL H2O. The phases were separated, and the aqueous phase extracted with another 75 mL CH2Cl2. The organic fractions were combined, washed first with H2O and then with saturated brine. Removal of the solvent under vacuum yielded an off-white oil that was distilled at 112-125 °C at 0.4 mm/Hg to provide 7.5 g of 3,4,5-triethoxybenzyl chloride that spontaneously crystallized. The crude product had a mp of 34-37 °C which was increased to 37.5-38.5 °C upon recrystallization from hexane. Anal. (C13H19ClO3) C,H.
A solution of 4.5 g 3,4,5-triethoxybenzyl chloride in 10 mL DMF was treated with 5.0 g sodium cyanide and heated for 1 h on the steam bath. The mixture was then poured into 100 mL H2O and the oily phase that resulted immediately crystallized. This was filtered off, washed well with H2O, air dried, and distilled at 128-140 °C at 0.25 mm/Hg to yield 3.7 g of 3,4,5-triethoxyphenylacetonitrile which melted at 54-56.5 °C. There was a sharp nitrile band at 2249 cm-1. Anal. (C14H19NO3) C,H.
To 18.8 mL of a 1 M solution of LAH in THF under N2 , vigorously stirred and cooled to 0 °C, there was added, dropwise, 0.50 mL 100% H2SO4. This was followed by 3.6 g 3,4,5-triethoxyphenylacetonitrile in 10 mL anhydrous THF over the course of 5 min. The reaction mixture was brought to room temperature and stirred for a few min, and finally held at reflux on the steam bath for 1 h. After cooling back to room temperature, there was added about 2 mL IPA (to destroy the excess hydride) followed by sufficient 15% NaOH to make the aluminum oxide granular and white, and the organic solution basic. The solids were removed by filtration, and washed with IPA. The filtrate and washes were stripped of solvent under vacuum, the residue added to 400 mL dilute H2SO4. This was washed with 2x75 mL CH2Cl2, the aqueous phase made basic with aqueous. NaOH, and the product extracted with 2x75 mL CH2Cl2. These extracts were pooled, the solvent removed under vacuum, and the residue distilled at 115-135 °C at 0.4 mm/Hg to give a white oil. This was dissolved in a few mL of IPA, neutralized with concentrated HCl, and diluted with anhydrous Et2O to the point of turbidity. When the crystal formation was complete, the product was removed by filtration, washed with Et2O, and air dried to give 2.8 g 3,4,5-triethoxyphenethylamine hydrochloride (TRIS) as white crystals with a mp of 177-178 °C.
DOSAGE: greater than 240 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 240 mg) No effects were noted at any time following 240 milligrams of trisescaline. This would have been a thoroughly active level of the trimethoxy counterpart, mescaline.
EXTENSIONS AND COMMENTARY: With the progressive diminution of human potency with increased ethylation of the mescaline molecule, there is no suprise in finding that this base is devoid of activity. Studies done years ago in the cat at a dosage of 25 mg/Kg (i.m.) gave none of the expected, and looked for, signs of behavioral changes (pilomotor activity, pupillary dilation, growling, hissing, aggressive behavior, withdrawal, or salivation) that are often seen with the less bulky substituents. It was without action.
More lengthy substituents in the 3,4,5-positions (with combinations of ethyls and propyls, for example) are presently unknown compounds, and there is small incentive to make them.
| [ |
[Main Index] | [Forward |

